–
金沢大学新学術創成研究機構の小川数馬教授、三代憲司准教授、大学院医薬保健学総合研究科薬学専攻/日本学術振興会特別研究員(DC1)(次世代精鋭人材創発プロジェクト令和 4 年度採用選抜学生)の平田咲(博士課程 2 年)、医薬保健研究域薬学系の淵上剛志准教授、宗兼将之助教、医薬保健研究域医学系(附属病院核医学診療科)の絹谷清剛教授、福島県立医科大学の高橋和弘教授、鷲山幸信准教授、千葉大学の荒野泰名誉教授らの共同研究グループは、生体内において芳香環(※1)上のアスタチン-211 (211At)(※2)を安定に保持する新規標識部位の開発を行いました。
α 線は非常に高い細胞傷害性を持ち、その飛程(※3)が短い特徴があります。そこで、α 線放出核種(※4)を持つ薬剤をがん細胞にのみに届けることで、副作用を抑えながら高い治療効果を得ることができます。特に 211At は、国内で製造が可能なほぼ唯一の α 線放出核種であり、近年その医療応用が注目されています。これまでの研究では、芳香環上に 211At を導入した薬剤が多く開発されてきましたが、芳香環上の 211At は生体における安定性が低く、薬剤構造から 211At が脱離することにより正常組織に意図せず集積することが課題となっていました。本研究では、芳香環上の 211At 周辺の構造修飾により、211At の脱離を抑制する技術を開発しました。異なる性質を持つ種々の置換基(※5)を導入した構造を検討した結果、211At を生体内で安定に保持できる標識技術の確立に成功しました。この技術は、より効果的で副作用の少ない核医学治療の実現につながることが期待されます。
本研究成果は、2025年1月5日アメリカ化学会が出版する国際誌『Journal of Medicinal Chemistry』のオンライン版に掲載されました。
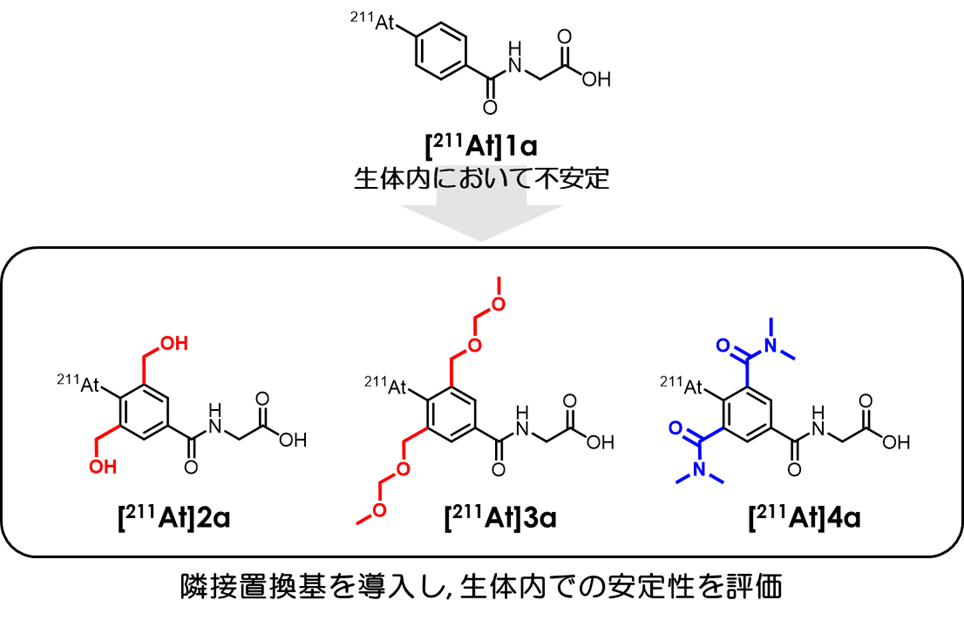 図:本研究の概念図
図:本研究の概念図
【用語解説】
※1:芳香環
ベンゼン環などの環状の構造のこと。医薬品、化粧品、香料などをはじめとする、生活に関わる多くの物の成分として利用される化学物質の構造に含まれている。
※2:アスタチン-211 (211At)
α 線放出核種の一つ。高い細胞傷害性を示すことから、がんに集めることにより、強力ながん治療効果が期待されている。ハロゲン元素であるため、ヨウ素や臭素など他のハロゲン元素と類似した化学的性質を持ち、既知の標識法を応用できる。
※3:飛程
放射線がエネルギーを失って(与えて)停止するまでの距離のこと。α 線の組織内の飛程は 20–100 µm、β−線の飛程は数 mm 程度である。
※4:α 線放出核種
α 線は、2 個の陽子と 2 個の中性子から構成されるヘリウム原子核であり、異なった原子に変化する時に α 線を放出する放射性核種のこと。
※5:置換基
有機分子の部分構造を構成する原子もしくは原子団のこと。ある母体化合物の水素原子を別の原子または原子団に置き換えた分子において、置き換えた原子または原子団を置換基と呼ぶ。
ジャーナル名:Journal of Medicinal Chemistry
研究者情報:小川 数馬




 PAGE TOP
PAGE TOP