金沢大学理工研究域物質化学系の片岡邦重 教授、山下哲 准教授、金沢大学大学院自然科学研究科の竹井利忠 博士前期課程学生、西山琢巳 博士前期課程学生(当時)、京都大学大学院農学研究科の足立大宜 特定研究員、宋和慶盛 助教、加納健司 名誉教授らの共同研究グループは、大腸菌由来の銅排出酸化酵素(CueO)(※1)における直接電子移動型酵素電極反応(DET型反応)(※2)を解析し、銅イオンの結合が引き起こす活性調節機構を解明しました。
CueOは、細胞内の銅恒常性を維持するため、毒性の高い1価銅イオン(Cu⁺)を2価銅イオン(Cu²⁺)へと酸化する重要な役割を担っています。また本酵素は、電極から電子を受け取り、酸素を水へと還元できるというユニークな特徴を有しており、DET型反応が可能な酵素(DET型酵素)としても注目されています。私たちは先行研究で、CueOのDET型反応がCu²⁺存在下で還元的不活性化(※3)を受けることを発見していましたが、その分子機構は明らかではありませんでした。
本研究では、CueOの構造情報に基づき、第8の銅(Cu8)結合部位が不活性化の鍵を握ると仮説を立てました。そこで、Cu8結合への関与が推定されるアミノ酸残基を置換することで、銅結合能を喪失させた変異体を作製し、その特性を電気化学的に評価しました。
金沢大学の研究グループは、Cu8が不活性化に関与する仮説設定と、検証に用いる変異体の作製を担当しました。すると、ヒスチジン残基を置換した変異体において、不活性化の大幅な抑制が確認されました。また速度論解析(※4)の結果、変異によってCu8の結合能と標準酸化還元電位の両方が変化することが明らかになりました。さらに、Cu8による不活性化は溶液中の酵素反応でも観測され、生体内でもCu²⁺/Cu⁺比を調節する仕組みとして機能している可能性が示唆されました。
本研究成果は、銅代謝制御機構への理解を深めるとともに、DET型酵素の高機能化に向けた分子設計指針を提供するものであり、生化学および電気化学分野への波及効果が期待されます。
本研究成果は、2025年12月11日に、国際学術誌『Inorganic Chemistry』にオンライン掲載されました。
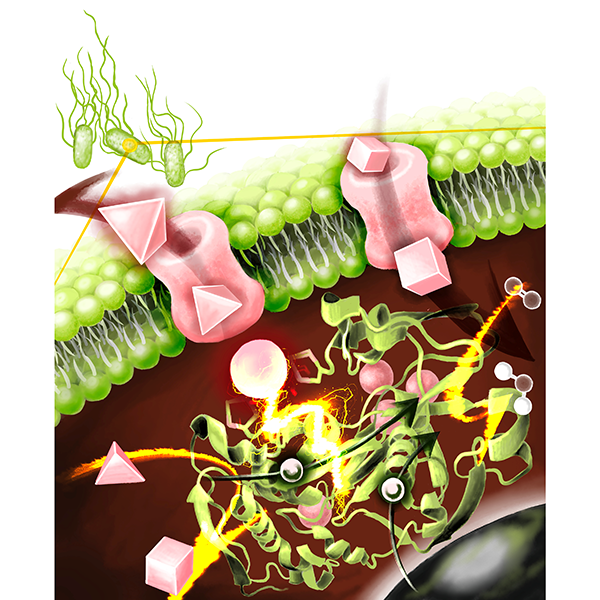
CueOが制御する細胞内銅代謝のイメージ
【用語解説】
※1 銅排出酸化酵素(CueO)
Cu⁺をCu²⁺へと酸化する反応、および酸素を水へと還元する反応を触媒する酵素。
※2 直接電子移動型酵素電極反応(DET型反応)
酵素反応と電極反応が共役した反応を「酵素電極反応」と呼びます。その中でも、酵素が電極と直接的に電子移動できるものを直接電子移動型と呼び、本文中ではDET型反応と記載しています。
※3 還元的不活性化
還元的環境、すなわち低電位において、酵素活性が低下する現象。その逆である酸化的不活性化が確認されている酵素もあります。
※4 速度論解析
理論式に基づく実験データの回帰分析。速度論解析により、反応に関わる各種パラメータを定量的に推定できます。
ジャーナル名:Inorganic Chemistry
関連情報
金沢大学 理工学域 物質化学類:https://chemistry.w3.kanazawa-u.ac.jp/
金沢大学大学院 自然科学研究科 物質化学専攻:https://www.nst.kanazawa-u.ac.jp/labp/chemistry/






 PAGE TOP
PAGE TOP