Abstract: Researchers from Kanazawa University synthesized sterically hindered ethers from acetals by using radical chemistry, enabled by a cheap and readily accessible titanium reagent. Such chemistry will be indispensable to expanding the chemical reaction conditions that are compatible with synthesizing complex pharmaceuticals, pesticides, and other advanced chemical compounds.
Kanazawa, Japan – Since as far back as the 1850s, synthesizing ethers has involved the same fundamental chemistry. Now, researchers from Japan have expanded the synthetic toolkit for ethers—by using cheap reagents and easy procedures.
In a study recently published in the Bulletin of the Chemical Society of Japan, researchers from Kanazawa University have revealed a new chemical synthesis of carbon radicals from acetals and have produced a wide range of ethers from otherwise challenging starting materials.
Ethers are an exciting class of chemical compounds that range from humble chemical solvents to stabilizing components of some recent COVID-19 vaccines. A type of chemical reaction known as nucleophilic substitution continues to be the predominant method for producing ethers. This has restricted the scope of the possible ethers one can form. Accordingly, researchers have focused on making use of chemical reaction intermediates known as carbon radicals. At present, such chemistry has not been well-developed in the context of ether synthesis from acetals, something the researchers at Kanazawa University aimed to address.
"We recently discovered an inexpensive titanium reagent that will be useful for expanding the scope of ether synthesis," says lead and co-senior author of the study Takuya Suga. "This idea has literature precedence, but to date, undesired chemistry has limited the practical applications."
To overcome prior technical challenges, the researchers used chemistry that they had previously applied to forming carbon–carbon bonds. The reaction conditions used here are benign even for the notoriously challenging chemistry of carbon radicals.
"Results from extensive chemical screening were clear," explains Yutaka Ukaji, co-senior author. "We obtained high yields of products, without extensive undesired side products, by carefully tailoring the composition of the titanium reagent, reductant, and additive."
Furthermore, the reaction even produced ethers from starting materials that have many large chemical units near the reaction center. It is otherwise notoriously challenging to synthesize ethers from such starting materials.
"We carried out a representative ether synthesis on a gram scale," says Suga. "This is important for demonstrating that our method is reliable and has real-world applications."
Cosmetics, pharmaceuticals, and many other products depend on the properties enabled by ethers. The results presented here are especially exciting because titanium is a cheap and abundant element, and the researchers' reagent can be synthesized from commercially available precursors. Further development will dramatically expand the scope of ethers that can be synthesized by using mild reaction chemistry.
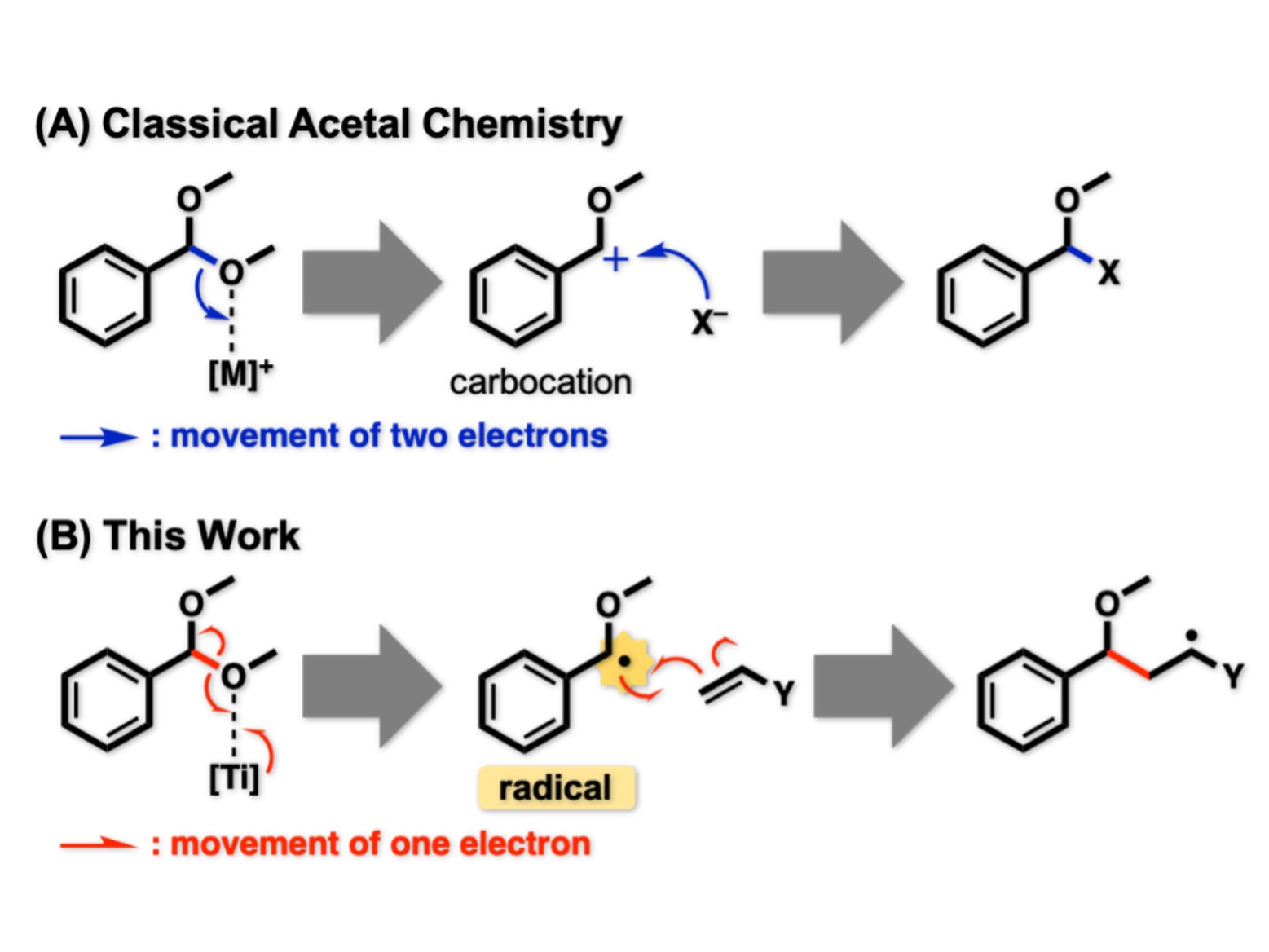
Figure 1. Activation mode of acetals.
(A) Classically, acetal C–O bonds are cleaved in heterolytic fashion. Two electrons constituting one C–O bond are both removed by an acidic activator ([M]+) to furnish carbocation. This has been utilized for nucleophilic substitution. (B) In the new strategy, acetal C–O bonds are cleaved in homolytic fashion. Because the reacting C–O bond and [Ti] provide one electron each other, an unpaired electron (•) is left on the substrate. This active species, benzyl radical, exhibits distinctive reactivity derived from the unpaired electron.
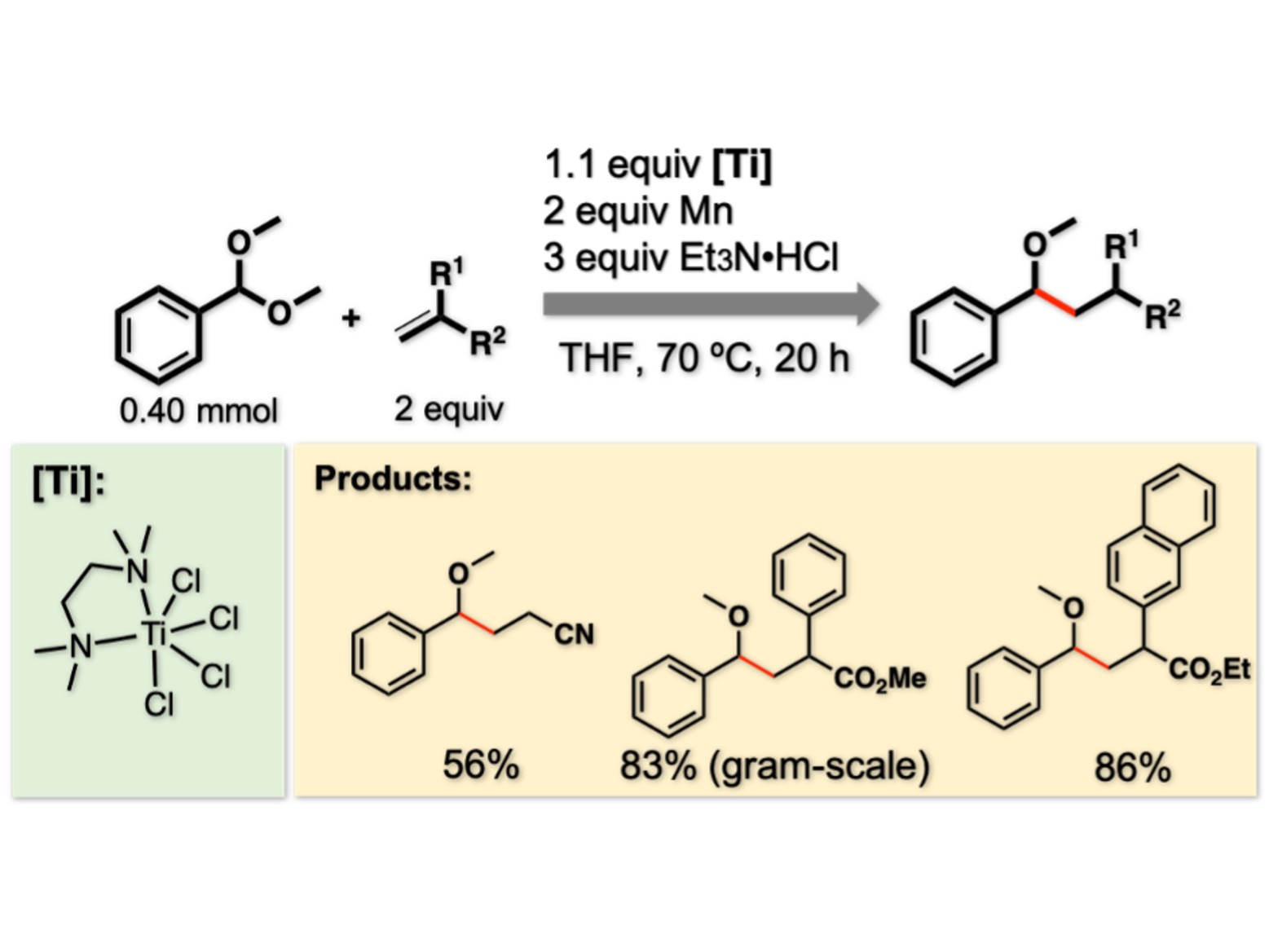
Figure 2. Conditions and examples of reactions.
The generated benzyl radical was utilized for C–C bond formation reactions with alkenes. A preceding reaction between TiCl4(tmeda) (shown Ti reagent) and Mn affords a low-valent Ti species which is responsible for the C–O cleavage. Et3N•HCl provides one hydrogen atom and terminates the reaction process.
[Funder]
This work was supported in part by Mitsui Chemicals Award in Synthetic Organic Chemistry, Japan; UBE Industries Foundation Award; Hokuriku Bank Research Grant for Young Scientists; and the Kanazawa University SAKIGAKE project.
[Article]
Title: Conjugate addition of acetal-derived benzyl radicals generated from low-valent titanium-mediated C–O bond cleavage
Journal: Bulletin of the Chemical Society of Japan
Authors: Takuya Suga, Masaharu Nakamura, Ryusei Takada, and Yutaka Ukaji



 PAGE TOP
PAGE TOP