Abstract:Researchers at Kanazawa University report in Chemical Communications how solvents influence the strength of donor–acceptor interactions. They found that silicone solvents, providing low compatibility, intensify donor–acceptor interactions between aromatic molecules compared to hydrocarbon solvents.
Aromatic donor–acceptor (D–A) interactions are a type of non-covalent bond between a donor (electron-rich) and an acceptor (electron-deficient) aromatic molecules. Aromatic molecules feature one or more rings with ‘delocalized’ electrons. The aromatic D–A interactions are widely used for building supramolecular structures, which are assembly of molecules formed by non-covalent bonds like building blocks. The supramolecular structures have smart properties such as external stimuli-responsiveness and self-repairing. The stability and smart properties of supramolecular architectures formed by D-A interactions depend on characteristics of the D–A interactions in various circumstances. Shogo Amemori and colleagues from Kanazawa University have now investigated the solvent effects on a strength of D–A interactions. Their findings provide important insights into the origin of aromatic D–A interactions and new molecular design of supramolecular architectures.
The researchers worked with organic compounds known as pyromellitic diimide (PMDI) and pyrene (Py) derivatives as acceptors and donors, respectively. The strength of the D–A interaction between the PMDI and Py derivatives was evaluated by the association constant of the D–A interaction, in which a higher association constant means stronger D–A interactions.
Amemori and colleagues used solvents with a variety of polarity for the evaluation of solvent effect on the D-A interaction. Polarity is related to the separation of charge (into positive and negative components) within a molecule; in low-polarity molecules, the charge separation is small. As most previous studies focused on low-polarity solvents belonging to the class of aliphatic molecules, the scientists explored another class of compounds, the so-called poly(dimethylsiloxanes) (PDMS) or oligo(dimethylsiloxane) (ODMS). These molecules consist of backbone of silicon-oxygen bonds fully covered organic groups, and, as solvents, generally lead to low-polarity.
The main finding of the researchers is that the siloxanes lead to higher association constants of the D–A interactions between PMDI and Py derivatives compared to that of hexane as aliphatic solvents. As to the origin of this effect, the scientists point to the ‘incompatibility’ between the aromatic molecules (PMDI and Py) and the siloxane molecules.
Amemori and colleagues point out that further investigation using various donor and acceptor molecules is necessary. Nevertheless, the result that D–A interactions are stronger in PDMS and ODMS solvents than in aliphatic solvents, possibly due to the generally poor solubility — quoting the scientists — “may help elucidate the origin of aromatic D–A interactions in low-polarity solvents”.
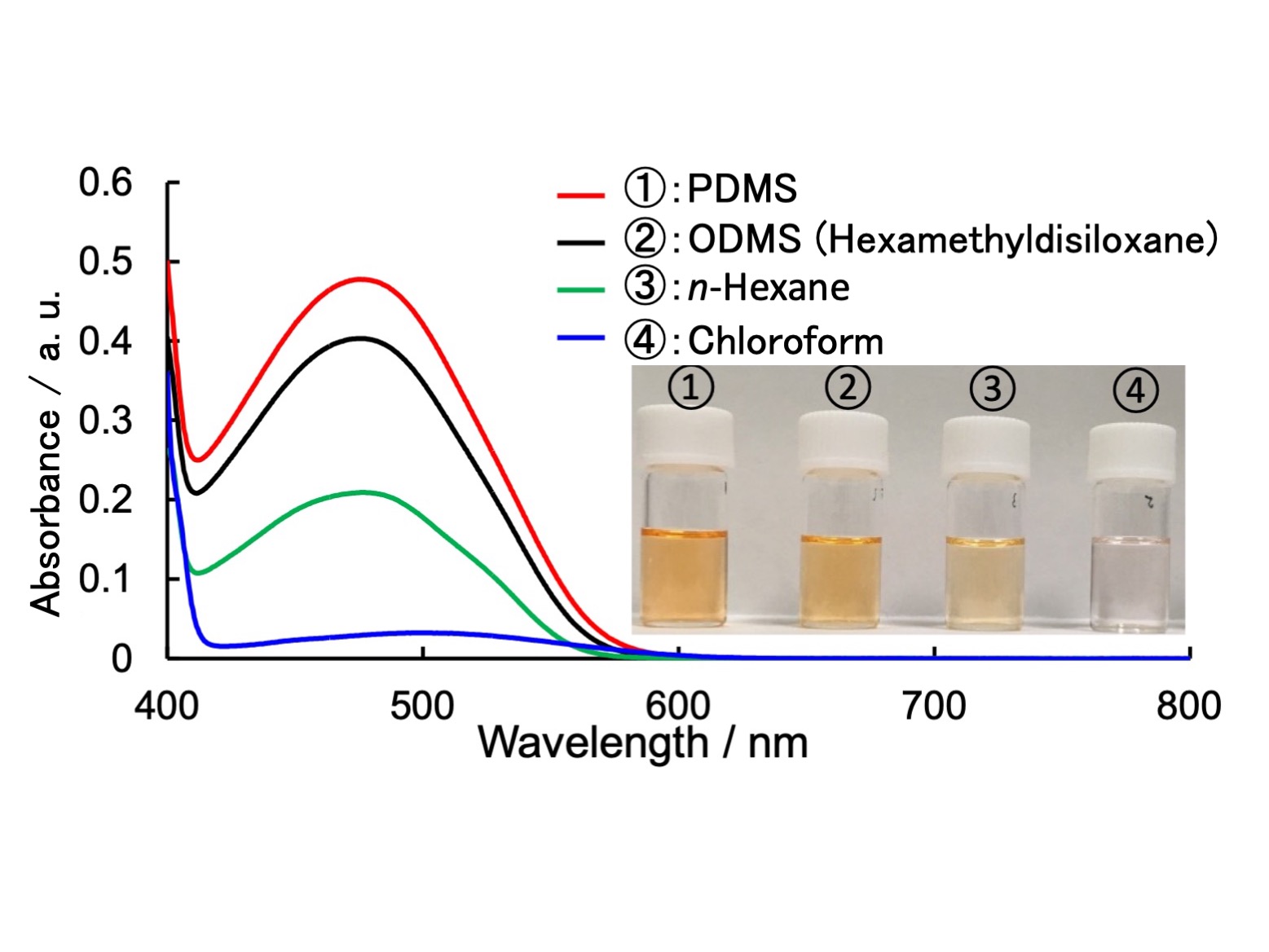
Figure.
Absorption spectra and photographs of mixtures containing Py and PMDI in PDMS, ODMS(hexamethyldisiloxane), n-hexane and chloroform (Concentration of Py and PMDI are 11 mM and 2 mM, respectively) . Darker color of solutions and a higher absorbance represent a higher association constant.
[Background]
Siloxanes
Siloxane refers to the functional group characterized by the silicon–oxygen–silicon (Si–O–Si) bonds. Siloxane-based compounds are human-made and, due to their hydrophobicity, high flexibility and low thermal conductivity, have many industrial and commercial applications. Siloxanes are the basis for silicones, materials used as e.g. adhesives, lubricants and sealants. A prominent member of the silicone family is poly(dimethylsiloxane) (PDMS); it is the most widely used silicon-based organic polymer. Shogo Amemori and colleagues from Kanazawa University used PDMS and shorter versions of it called oligo(dimethylsiloxane) (ODMS) as low-polarity solvents for aromatic donor and acceptor molecules that were let to interact with each other. They found that because of the incompatibility between the aromatic molecules and the siloxanes, the interactions were stronger than with other low- polarity solvents, non-siloxane-based solvents, such as hexane.
[Article]
Title: Poly(dimethylsiloxane) and oligo(dimethylsiloxane) solvent effects on aromatic donor–acceptor interactions
Journal: Chemical Communications
Authors:Shogo Amemori, Kyoka Kikuchi and Motohiro Mizuno
[Funder]
This work was supported by JSPS KAKENHI Grants 18K14187 (Early-Career Scientists).



 PAGE TOP
PAGE TOP