Abstract:The synthesis of formate, an energy carrier, from CO2 using electricity derived from renewable sources is attracting much attention as a technology for recycling and reusing CO2. Here, we focus on reduced graphene oxide (rGO) as a dispersed Sn support that efficiently adsorbs CO2. Highly efficient synthesis of formate is observed at the interface between rGO and Sn in the electrochemical reduction of CO2. We propose a reaction mechanism for this electrochemical reduction.
[Background]
Decreasing the emission and efficient utilization (fixation) of carbon dioxide (CO2) are worldwide issues to prevent global warming. Promotion of the use of renewable energy is effective in reducing CO2 emissions. However, since there are large time-dependent fluctuations and large regional differences in renewable energy production, it is necessary to establish a fixation technology to allow efficient energy transportation and storage. Thus, there is increasing interest in technologies for synthesizing useful chemicals from CO2 using electricity derived from renewable energy. In particular, formic acid is attracting much attention as an energy (hydrogen) carrier because it is liquid and non-toxic at room temperature. Establishment of this technology will contribute to the efficient transportation and storage of renewable energy and to the fixation of CO2, and allow energy storage with high environmental compatibility to be achieved.
In the electrochemical reduction of CO2, it is known that formic acid can be obtained with a Faradic efficiency*1) of about 50 to 60% by using tin (Sn) as a cathode catalyst. However, in order to develop this technology for practical use, further improvement of Faradic efficiency and a reduction of overpotential*2) are necessary. There is much active interest in research to understand the design principles of catalysts to enable these goals to be achieved.
[Results]
The present research team led by Prof. Tsujiguchi and his colleagues of Kanazawa University in collaboration with scientists from University of Tsukuba and Osaka University prepared a tin/reduced graphene oxide*3) (Sn/rGO) catalyst in which Sn was supported on reduced graphite oxide by thermal reduction of tin chloride (SnCl2) and graphene oxide (GO) obtained by oxidizing graphite powder using the improved Hummers method*4). In the catalyst thus prepared, Sn is uniformly dispersed in the rGO layer, and the composite is stacked to form a 3D morphology, i.e. rGO/Sn/rGO (Fig. 1). This catalyst is characterized as a carrier with functional group containing a much larger amount of oxygen than the tin/graphite catalyst used for a comparison. When we performed the electrochemical reduction of CO2 using these catalysts with CO2 dissolved in a solution of potassium hydrogen carbonate (KHCO3), it was found that the Sn/rGO catalyst significantly decreased the overpotential and allowed a high current density to be obtained compared to the Sn catalyst. In addition, when the reduction of CO2 was performed at a constant potential, almost no products other than formic acid, such as H2 and CO, were detected and we succeeded in obtaining formic acid with a Faradic efficiency of 98% (1.8 times that with Sn catalyst alone) (Fig. 1).
The reason for the highly efficient formic acid production obtained using Sn/rGO catalyst is its high CO2 adsorption capacity. Sn/rGO can adsorb four times as much CO2 as Sn catalyst alone. Further, the rate of CO2 adsorption is eight times that of Sn catalyst alone. Computational chemistry predicted that this high CO2 adsorption capacity would be due to the oxidized functional groups of rGO and that the production of hydrogen and carbon monoxide would be suppressed since the CO2 adsorbed by the oxidized functional group of rGO is quickly and efficiently supplied to the adjacent Sn surface (Fig. 2). In order to experimentally confirm this mechanism, our team attempted electrochemical imaging of the catalytic activity with a scanning electrochemical cell microscope*5). It was revealed that a significantly higher reduction current density was observed at the interface between Sn and rGO than on the Sn or rGO surfaces (Fig. 3) suggesting that a large amount of formic acid is synthesized on Sn adjacent to rGO, in support of the above prediction by computational chemistry. This is the first experimental demonstration using a scanning electrochemical cell microscope that formic acid synthesis is actively occurring at the interface between the catalyst and the support. Thus, more efficient formic acid synthesis would be possible by combining a support with a high CO2 adsorption capacity with a catalyst for electrochemical reduction of CO2*6). This provides an important framework that could be applied to all catalysts available so far.
[Future prospects]
The results of the present study provide new insights into the development of catalysts for formic acid synthesis by the reduction of CO2, and dramatic progress is expected in the development of formic acid synthesis technology by the electrochemical reduction of CO2. In addition, we have demonstrated an improvement of selectivity due to the excellent CO2 adsorption capacity of the support as well as elucidating its reaction mechanism. This should have a great impact on electrochemical reduction technology related to CO2, including the synthesis of methanol, methane and olefins. Therefore, it has the potential to be a useful basic technology in the synthesis of chemicals from CO2. In the future, we expect that the development of electrochemical reduction cells using this catalyst will be encouraged, leading to the creation of energy storage devices with high environmental compatibility that can contribute to the fixation of CO2 and promotion of the efficient use of renewable energy.
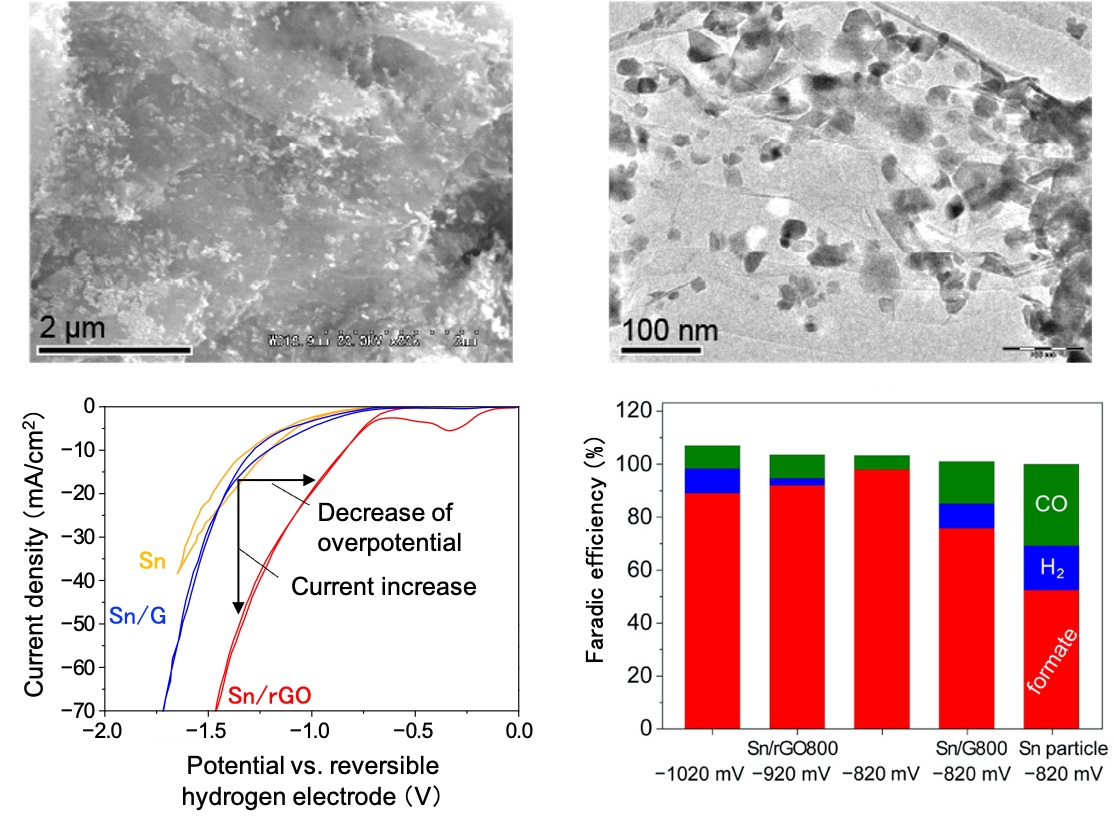
Figure 1.
Scanning electron microscope image (upper left), transmission electron microscope image (upper right), reduction characteristics (bottom left) and Faradic efficiency (bottom right) of Sn/rGO catalyst.
It can be seen that Sn nanoparticles of 10-50 nm are uniformly dispersed on the reduced graphene oxide sheet (upper left and upper right). Also, the absolute value of the current density under CO2 flow is larger than that of the conventional catalyst (Sn) or Sn supported graphene oxide (Sn/GO), and the current increases from an initial electric potential of a lower absolute value. Thus, it can be seen that the overpotential is significantly reduced and that the current density is augmented. In addition, the Faradic efficiency of formate is very high using the Sn/rGO catalyst (bottom left and bottom right).
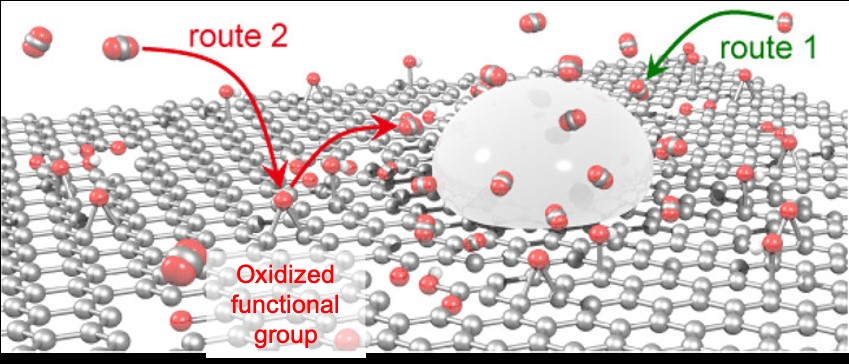
Figure 2. Conceptual scheme of CO2 adsorption onto the Sn/rGO catalyst.
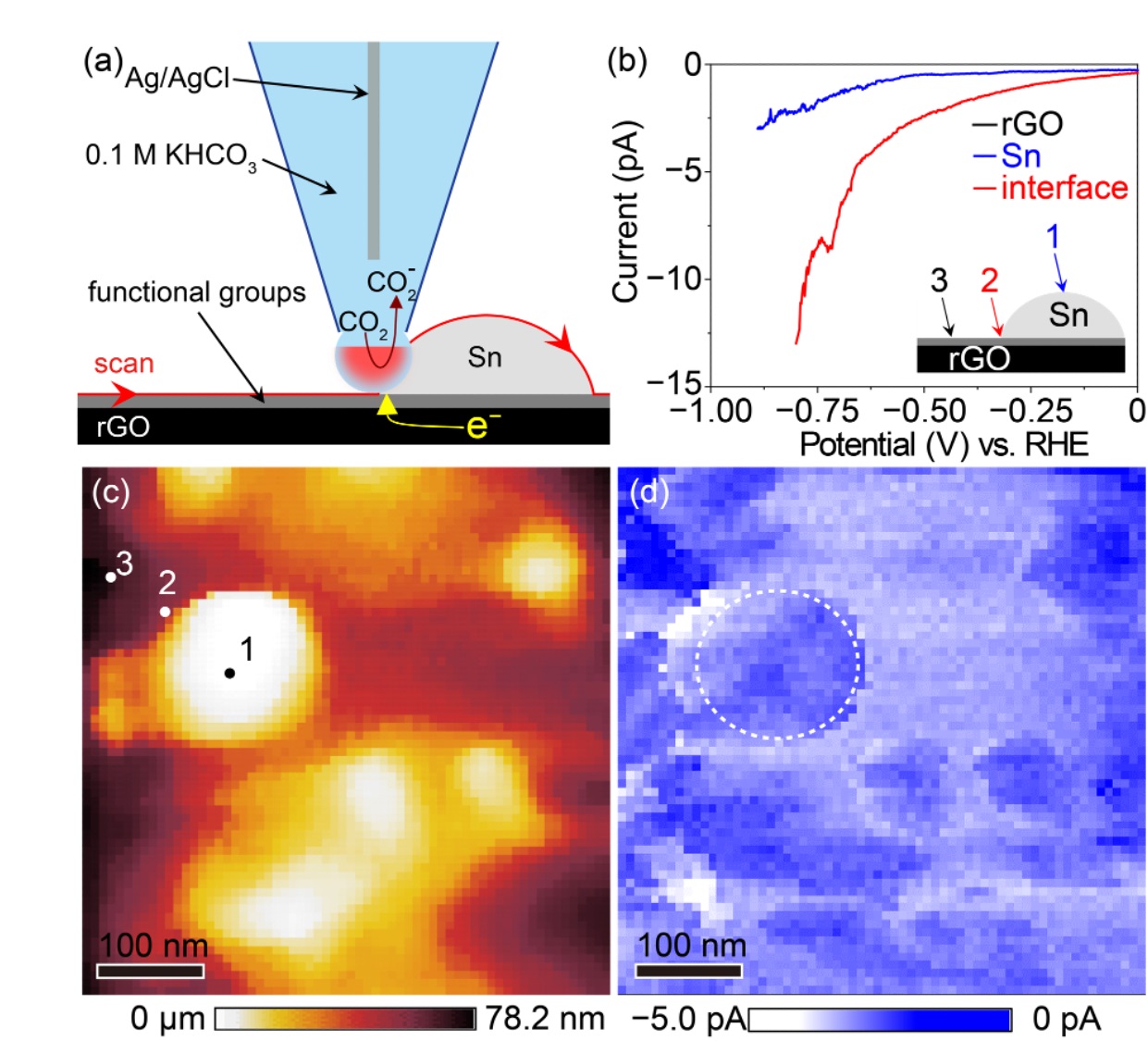
Figure 3.
[Glossary]
*1) Faradic efficiency
The ratio of the partial current that contributes to the synthesis of the object to the total current.
*2) Overpotential
The difference between the theoretically calculated equilibrium potential (voltage) and the actual potential (voltage) when the reaction takes place.
*3) Reduced graphene oxide
Graphene that is oxidized to have a functional group such as a hydroxyl, carboxyl, or epoxy group is called graphene oxide. The reduction product of graphene oxide is called reduced graphene oxide. While graphene oxide is an insulator, reduced graphene oxide is a conductor and is expected to be useful as an electrode material.
*4) Improved Hummers method
The Hummers method is a method in which graphite is used as a starting material, oxidized with potassium permanganate and sulfuric acid, peeled off as graphene oxide as a monoatomic layer, and then reduced to obtain a multilayer graphene sheet. The improved Hummers method is a modification of the Hummers method for the purpose of improvement of the yield of graphene oxide as a monoatomic layer or simplification.
*5) Scanning electrochemical cell microscope
A nanoscale electrochemical cell is formed on the sample surface using a nanopipette filled with an electrolyte, a meniscus-like electrochemical cell is formed between the nanopipette and the sample surface, and a local electrochemical measurement is performed. By scanning the nanopipette, an electrochemical image of the catalytically active site can be obtained.
*6) Electrochemical reduction of CO2
Technology that synthesizes useful chemicals by electrolyzing CO2. Various substances such as CO and methanol can be synthesized but the efficiency is currently poor.
[Article]
Title: Acceleration of Electrochemical CO2 Reduction to Formate at the Sn/Reduced Graphene Oxide Interface
Journal: ACS Catalysis
Authors:Takuya TSUJIGUCHI, Yusuke KAWABE, Samuel JEONG, Tatsuhiko OHTO, Suresh KUKUNURI, Hirotaka KURAMOCHI, Yasufumi TAKAHASHI, Tomohiko NISHIUCHI, Hideki MASUDA, Mitsuru WAKISAKA, Kailong HU, Ganesan ELUMALAI, Jun-ichi FUJITA, and Yoshikazu ITO
[Funder]
Japan Science and Technology Agency (JST) Strategic Creative Research Promotion Project PRESTO (JPMJPR18T), Ministry of Education, Culture, Sports, Science and Technology (MEXT) Scientific Research Grants (JP20H04628, JP20H04639, JP17H06460), Japan Society for the Promotion of Science (JSPS) Grants-in-Aid (JP15H05422, JP18K14174, JP19K15505), CRDA-IMR Tohoku University (20G0002), JSPS World Premier International Research Center Initiative (WPI).



 PAGE TOP
PAGE TOP