Abstract:
Hematopoietic stem cells (HSCs) can be used to better understand and treat blood-based diseases. Stem cell research in the model organism zebrafish is well-studied in the developmental stage, but is limited in the adult stage because HSCs are difficult to purify in this species. Researchers at Kanazawa University and their collaborators have developed a new purification scheme that allows HSCs to be purified from adult zebrafish kidneys, potentially opening new possibilities for stem cell research.
Kanazawa, Japan – Hematopoietic stem cells (HSCs) are multipotent cells that can develop into every type of blood cell in the body. They can also be used in medical research to understand and treat blood-based diseases. Zebrafish (Danio rerio) are used to study HSCs, particularly in the field of developmental biology, but the research in the adult animal is often limited because stem cells are difficult to purify in this species. Researchers at Kanazawa University and their collaborators now describe a purification scheme that allows these elusive zebrafish HSCs to be collected.
“Zebrafish are a great system to study how hematopoietic cells function in normal development and their role in disease,” says lead researcher Isao Kobayashi. “Much of their biology mirrors what we see in humans, and with zebrafish there’s the added benefit of having quite a few experimental tools at our fingertips, including live cell imaging and comparative analysis among vertebrates. Unfortunately, it’s proven challenging to effectively isolate HSCs from this species, and this has been a major impediment to the field.”
HSCs are highly abundant in the kidneys of adult zebrafish (unlike in humans, where HSCs are found in bone marrow). The challenge is separating them from other cells found in kidneys. Cell separation usually involves a purification technique called flow cytometry, where cells are sent in single file through a tube and hit with a laser beam. The machine (a flow cytometer) then sorts the cells based on how they reflect or scatter light.
In the study, published in Scientific Reports, the researchers created a strain of zebrafish that makes two light-emitting proteins, one green (Green Fluorescent Protein, GFP) and one red (mCherry), that can be sensed and sorted by a flow cytometer. Each fluorescent protein in this zebrafish strain was regulated by the genes related with blood cells, but the cells having both fluorescent proteins were limited in HSCs. By color coding the cells with two distinct blood cell markers, the team was able to purify cells that show hallmark signs “stemness” – like the ability to self-propagate and differentiate into other types of blood cells.
So, what might the successful isolation of HSCs in zebrafish mean for the field of stem cell research?
“When HSCs were finally purified in mice, the research community learned an enormous amount about how and where stem cells self-renew and differentiate to form blood cells,” says co-author Mao Kondo. “We’re very hopeful that this might spur a similar proliferation of research in zebrafish. In addition to some experimental advantages in zebrafish, we found that zebrafish HSCs share many key genes in common with HSCs in mammals. This suggests that mechanistic discoveries in zebrafish could have direct implications for understanding blood diseases in humans and for developing new medical treatments.”
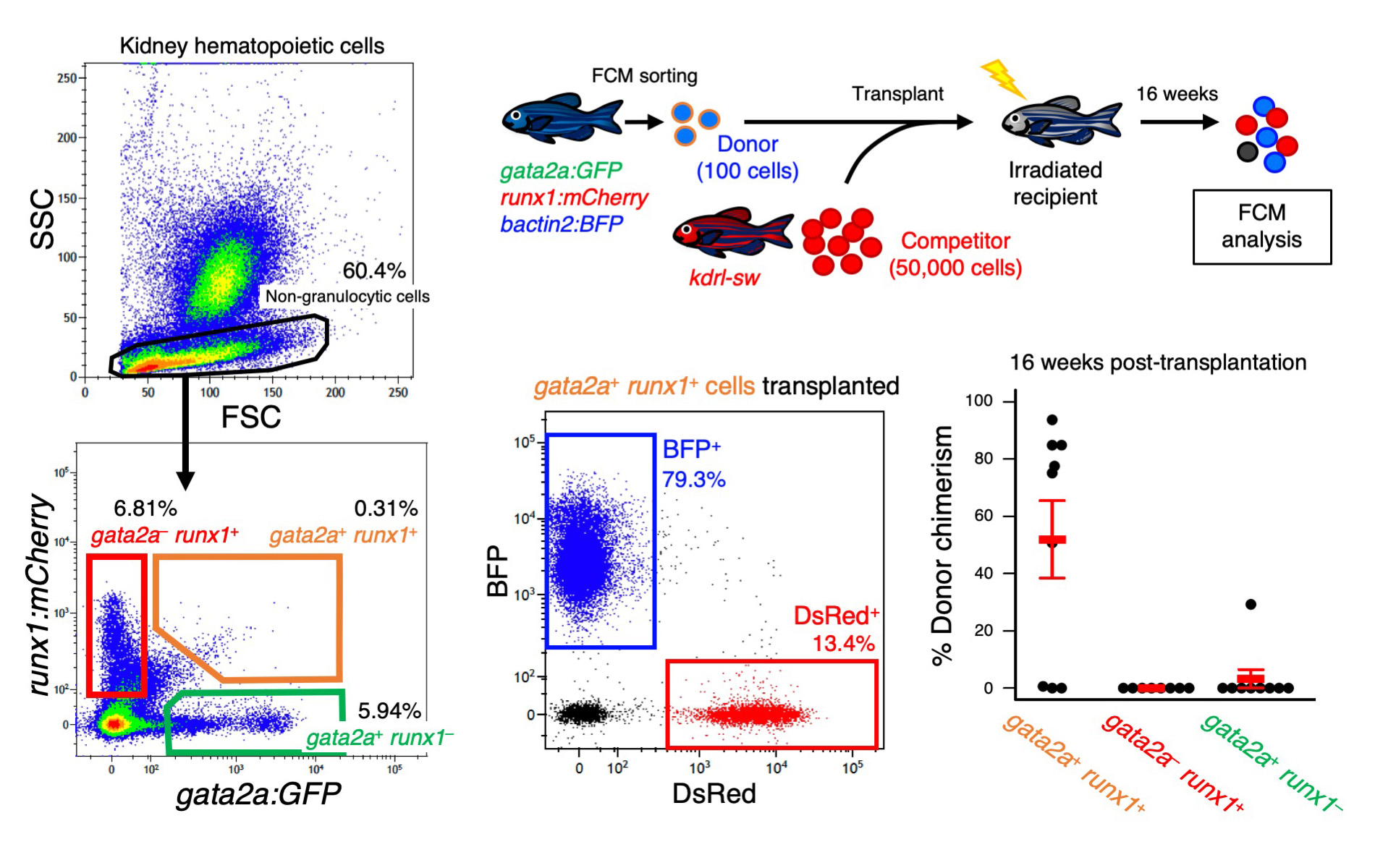
Figure.
Hematopoietic stem cells can be isolated as gata2a:GFP+ runx1:mCherry+ (gata2a+ runx1+) cells in the zebrafish kidney by flow cytometry (left panels). Transplantation assays confirmed the hematopoietic potential of gata2a+ runx1+ cells (right panels).
Article
Enrichment of hematopoietic stem/progenitor cells in the zebrafish kidney
Journal: Scientific Reports
Authors: Isao Kobayashi, Mao Kondo, Shiori Yamamori, Jingjing Kobayashi-Sun, Makoto Taniguchi, Kaori Kanemaru, Fumihiko Katakura & David Traver
DOI: 10.1038/s41598-019-50672-5
Funder
This work was supported in part by Grant-in-Aid for Young Scientists (B) from the Japan Society for the Promotion of Science (17K15393).



 PAGE TOP
PAGE TOP