Abstract:
Researchers from Kanazawa University have identified two key proteins, ASC and NLRP3, in the maintenance of the innate immune homeostasis in the airway. These proteins do so by a caspase-1-independent mechanism, suggesting that there may be multiple mechanisms involved in protection against microbial infections.
Kanazawa, Japan – Contrary to popular belief, there may be more than one mechanism involved in microbial airway protection. Researchers from Japan and China have found an alternative mechanism that may be involved in fighting microbial infections.
The inflammation pathway in response to microbial infections involves multiprotein complexes known as inflammasomes. It is known that inflammasomes induce inflammation by activating caspase-1, an enzyme that cleaves other proteins. In turn, caspase-1 converts the precursors of interleukin-1β and interleukin-18 known as pro-inflammatory cytokines to their active forms. In addition, caspase-1 processes gasdermin D, leading to pyroptosis, a necrotic form of inflammatory cell death.
In a study published in July of this year in Mucosal Immunology, the researchers found that caspase-1 is unnecessary in maintaining innate immune homeostasis in the airway. Instead, two protein components of the inflammasome, ASC and NLRP3, have found to be key players in this process.
“Based on previous reports, we understood that the protein NLRP3, plays a protective role against Streptococus pneumonia,” says study author Kohsuke Tsuchiya. “Hence, we decided to further investigate the role of NLRP3 in microbial protection. Using mice models, we found that NLRP3, along with ASC, may play a protective role against S. pneumoniae.”
The research team also found that ASC and NLRP3 protect the mice from S. pneumonia through a mechanism that is independent of caspase-1. Therefore, the researchers set out to elucidate this caspase-1-independent mechanism.
The results revealed that ASC and NLRP3 positively controlled the expression of the protein SPDEF during infection with S. pneumonia. SPDEF is a transcription factor involved with the expression of the mucosal defense factor genes. The study also showed that ASC and NLRP3 was key in activating STAT6, a regulator of the Spdef gene.
“Our results suggest that ASC and NLRP3 protect against microbial infections possibly through the STAT6-SPDEF pathway, which is independent of the caspase-1-dependent mechanism. This also means that both ASC and NLRP3 are acting independent of the inflammasome mechanism,” says author Rendong Fang.
The group’s findings may allow for new understanding of different possible mechanisms involved in maintaining innate immunity in the airway. However, further experiments are required, such as investigation into the mechanism linking S. pneumonia infection to STAT6 activation and whether both ASC and NLRP3 will maintain the expression of mucosal defense genes when infected by other microbial agents.
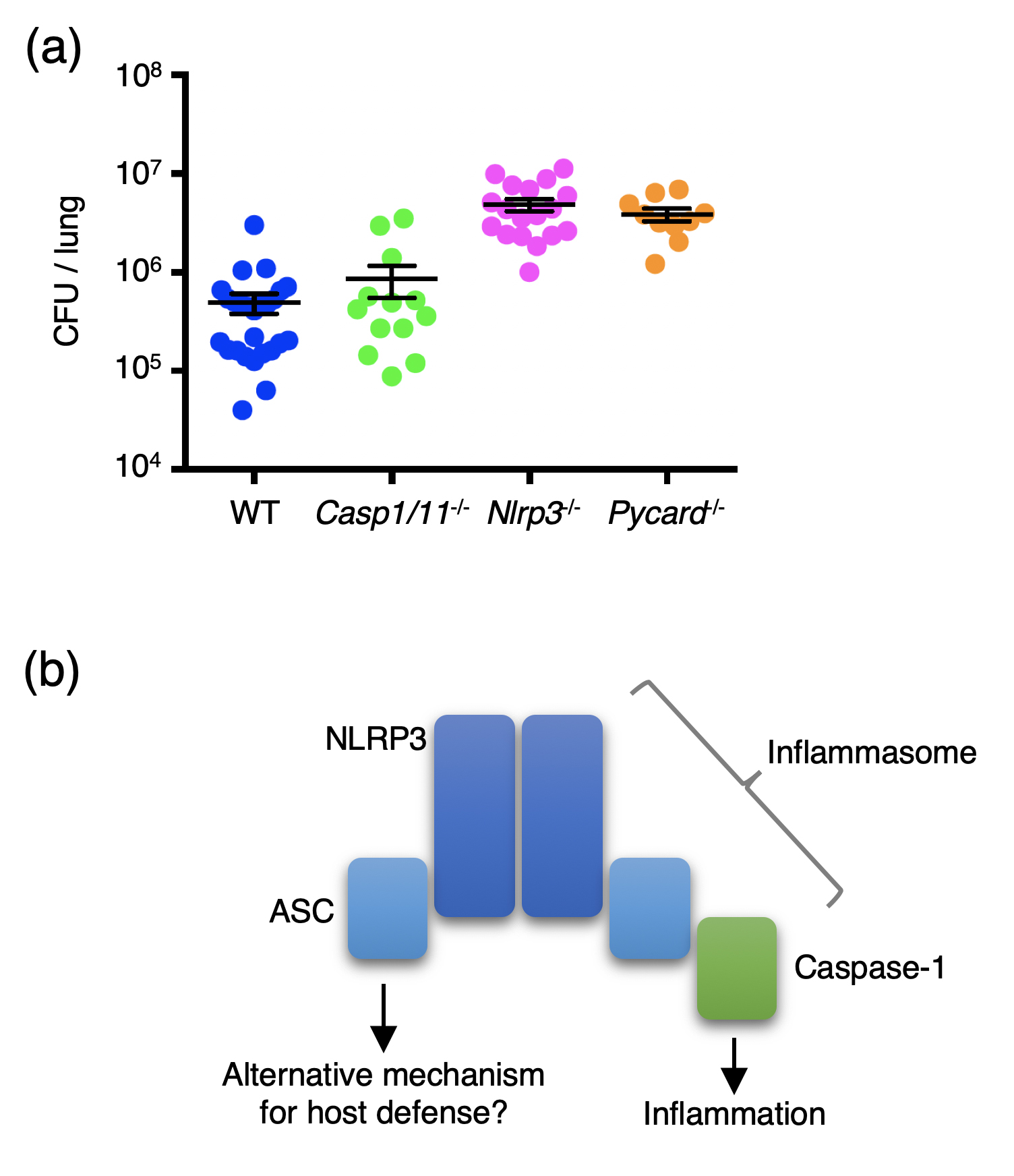
Figure 1.
(a) Mice of the indicated genotypes were infected with Streptococcus pneumonia intranasally, and CFU counts in the lung were determined 48 h after infection. Casp1/11-/-, Nlrp3-/-, and Pycard-/- mice are deficient in caspase-1, NLRP3, and ASC, respectively. The result suggests that NLRP3 and ASC, but not caspase-1, are required for host resistance to pneumococcal pneumonia.
(b) NLRP3 and ASC form the NLRP3 inflammasome in response to distinct stimuli, including S. pneumoniae. The inflammasome recruits and activates caspase-1, thereby promoting inflammation. However, NLRP3 and ASC seem to protect against pneumococcal pneumonia in a caspase-1-independent manner, suggesting an alternative mechanism for host defense that involves NLRP3 and ASC.
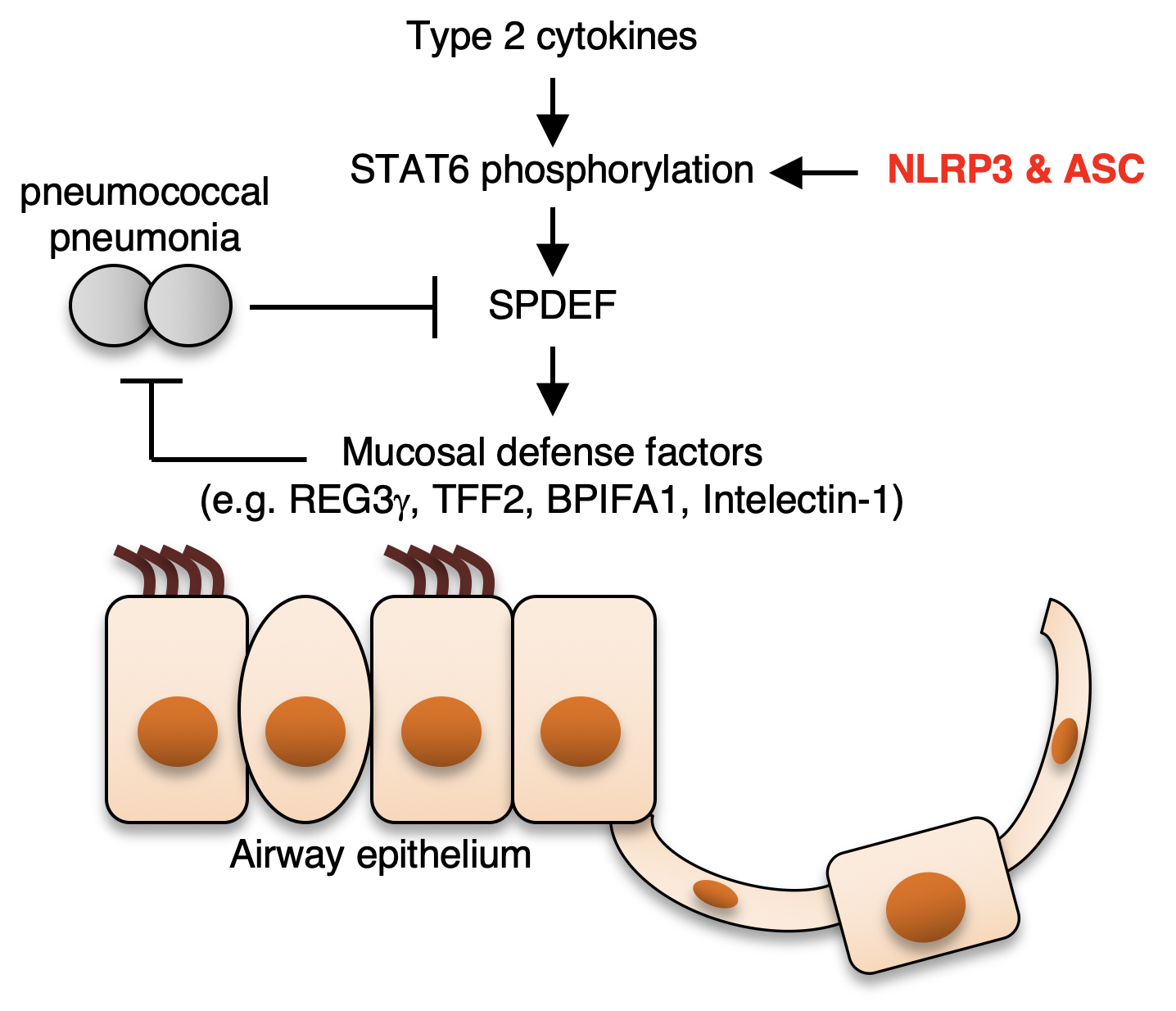
Figure 2.
ASC and NLRP3 are components of the NLRP3 inflammasome that promotes inflammation by activating caspase-1. However, ASC and NLRP3, but not caspase-1, are required for resistance to pneumococcal pneumonia, suggesting a protective role of ASC and NLRP3 independent of the inflammasome. This study aimed to elucidate the mechanism by which ASC and NLRP3 protect the host from pneumococcal pneumonia in an inflammasome-independent manner. Gene expression profiling revealed that the expression of mucosal defense factors, including TFF2 and intelectin-1, is maintained by ASC and NLRP3, but not caspase-1, in the airway during pneumococcal pneumonia. Mechanistically, ASC and NLRP3 maintain the expression of SPDEF, a transcription factor that positively regulates the expression of the mucosal defense factors, by enhancing STAT6 activation. Hence, ASC and NLRP3 are suggested to promote airway mucosal innate immunity by an inflammasome-independent mechanism involving the STAT6-SPDEF pathway.
Article
ASC and NLRP3 maintain innate immune homeostasis in the airway through an inflammasome-independent mechanism
Journal: Mucosal Immunology
Authors: Rendong Fang, Ryosuke Uchiyama, Shunsuke Sakai, Hideki Hara, Hiroko Tsutsui, Takashi Suda, Masao Mitsuyama, Ikuo Kawamura & Kohsuke Tsuchiya
DOI: 10.1038/s41385-019-0181-1
Funder
This work was supported by the Japan Society for the Promotion of Science (JSPS KAKENHI Grant Numbers 26460523, 18K07106, 15K15078, 26110002, and 24590522), the Ministry of Health, Labor and Welfare of Japan, the National Key Research and Development Program of China (2018YFD0500500) and National Natural Science Foundation of China (31400762).



 PAGE TOP
PAGE TOP