Abstract:
Hepatocyte growth factor (HGF) is involved in cancer progression through MET receptor signaling. Here, HiP-8, a 12 amino-acid macrocyclic peptide, was identified to selectively recognize active HGF. Biochemical analysis and high-speed AFM demonstrated HiP-8 restricted dynamic domains of HGF, resulting in allosteric inhibition. Positron emission tomography using radiotracer HiP-8 enabled noninvasive visualization and inhibition of HGF–MET activation status in tumors in mouse models. HiP-8 is expected to be useful for cancer diagnosis and treatment.
[Background]
Hepatocyte Growth Factor (HGF)*1) is a protein that acts as a cell growth factor. By binding to its receptor protein MET on the cell membrane, it exerts its physiological functions such as proliferation and migration of cells, as well as tissue repair and regeneration. For a cancer tissue, since it promotes invasion and survival of cancer cells, it can play roles in cancer metastasis and acquisition of resistance against anti-cancer drugs. HGF exists in a precursor form, inactive HGF, in various tissues, but the precursor is converted into active HGF only in the vicinity of cancer cells. Because of this reason, molecules that selectively detect/inhibit active HGF are highly desirable but have not been described so far.
Molecular-targeted drugs that selectively inhibit specific molecules have been developed and used for cancer treatment and for other diseases. Among others, antibodies show high specificity to their target molecules, but one of their drawbacks is their very high cost. On the other hand, synthetic drugs are much less expensive since they can be chemically synthesized but their specificity against their target molecules is not high. Recently, much attention has been paid to macrocyclic peptides*2), which may show target specificity as high as antibodies and yet may be available by chemical synthesis.
[Results]
The present collaborative research team from five institutions including Kanazawa University successfully discovered a macrocyclic peptide, HiP-8, which binds to the active form of HGF to inhibit its activity, using the PaPID method*3). HiP-8 is a macrocyclic peptide consisting of 12 amino acid residues in a ring-like geometry (Figure 1), binding to active HGF with a very high affinity and inhibiting HGF binding to its receptor MET (Figure 1).
In addition, in order to elucidate the functional interaction between HiP-8 and HGF, this study employed high-speed atomic force microscopy (HS-AFM)*4) developed at Kanazawa University, which allows visualization of the dynamics and morphology of biological molecules (Figure 2). This investigation successfully showed that, while an active HGF molecule exhibited dynamic behavior, upon binding of HiP-8, the molecular dynamics of HGF was strongly inhibited. This novel discovery shows that the highly dynamic nature of a protein molecule was inhibited by a small molecule.
Further, image analysis using positron emission tomography (PET)*5) and radio-labeled HiP-8, administered to mice xenografted with human lung cancer tissue, suggest a possible application of HiP-8 to cancer diagnosis. HiP-8 selectively accumulated in cancer tissue with active HGF (Figure 3), suggesting that HiP-8 should be useful as a probe for PET imaging diagnosis. Since HiP-8 inhibits HGF activity, it could also be used as an anti-cancer molecular targeted drug selective for active HGF.
[Future prospects]
The results of the present study are expected to lead to the development of new PET diagnostic methods with radiotracer HiP-8, a macrocyclic peptide. HiP-8 is a candidate molecule of a new molecular-targeted drug that selectively inhibits only active HGF.
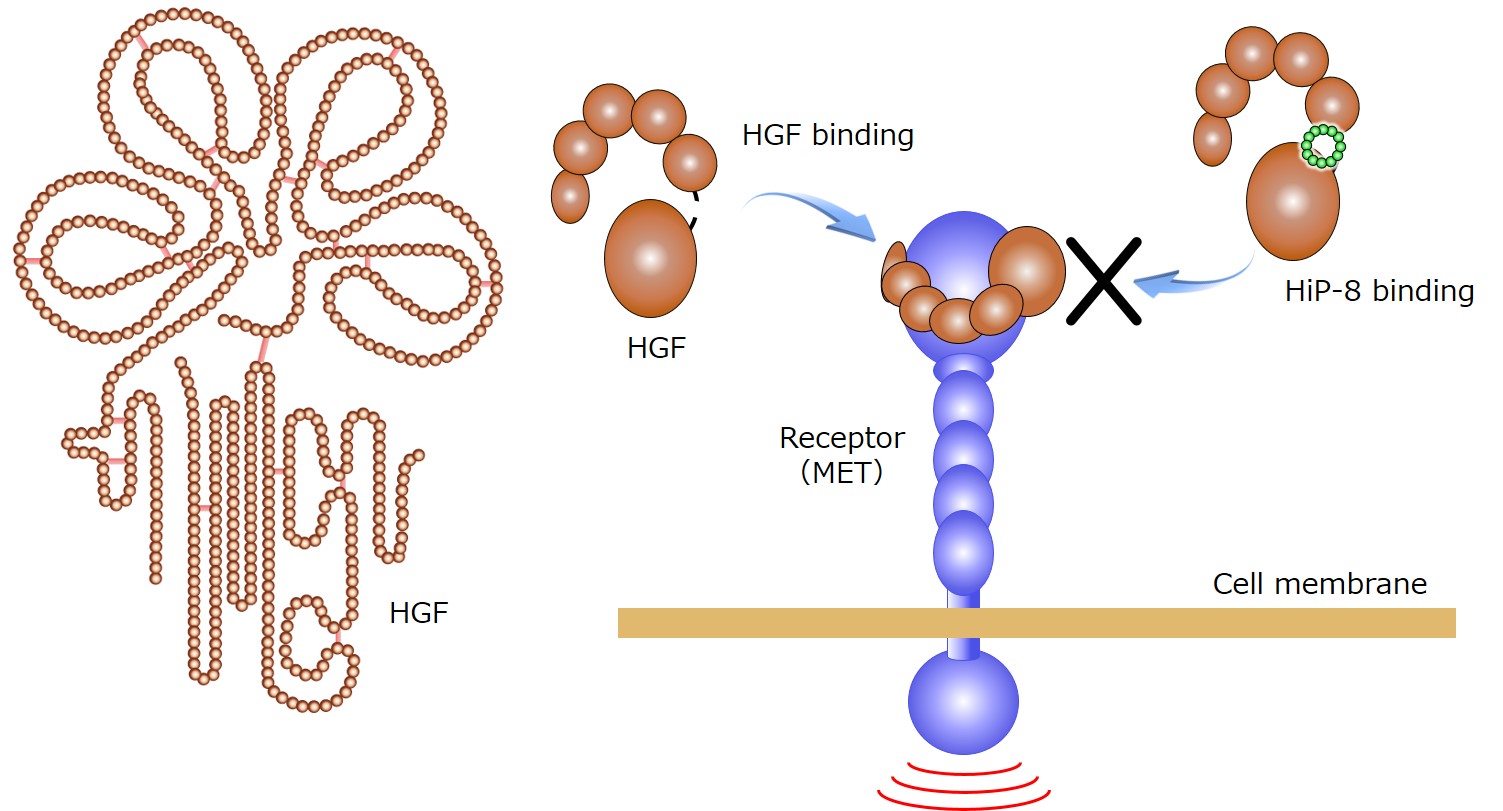
Figure 1. The outline of HiP-8 action.
HGF exerts its physiological functions by binding to its receptor MET on the cell membrane. Upon binding HiP-8 to HGF, HGF binding to the receptor MET is inhibited (right side of the Figure).
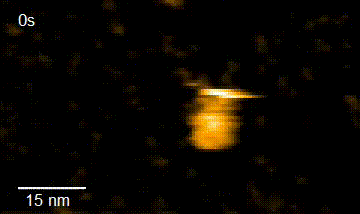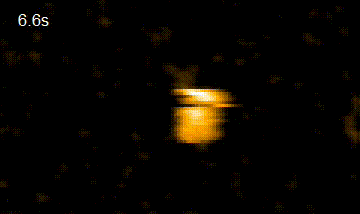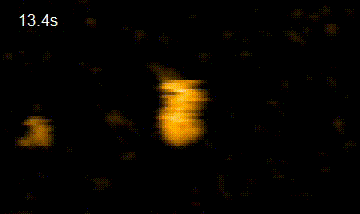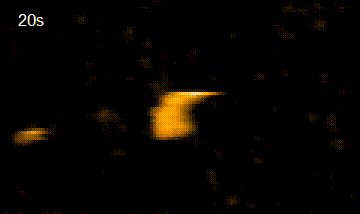

HGF HGF+HiP-8
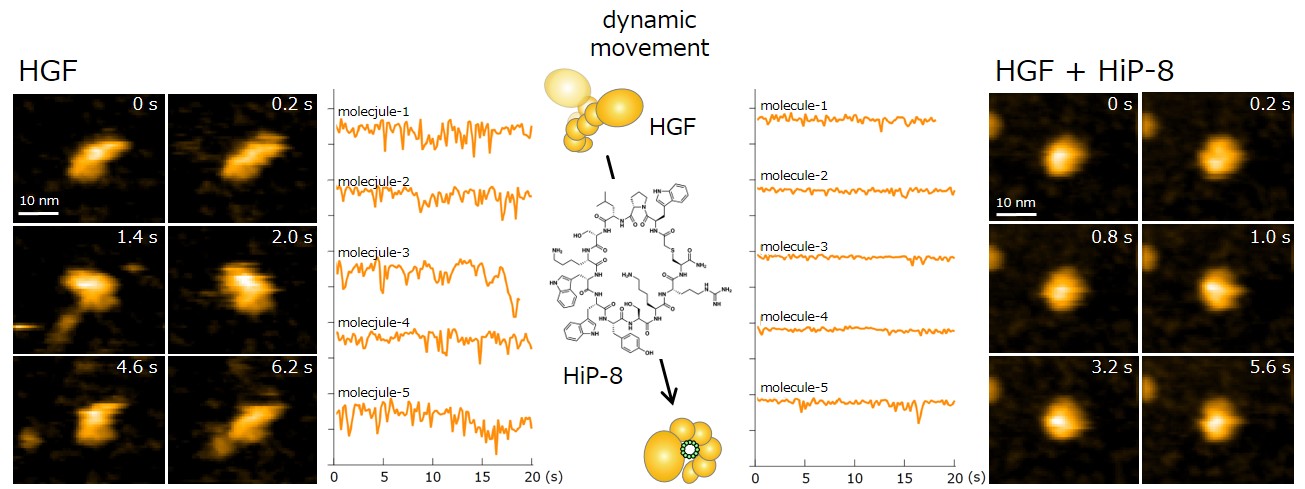
Figure 2. Observation using high-speed atomic force microscopy.
Yellow lines show change in molecular shape of HGF molecules. While HGF morphology undergoes dynamic motion, the dynamic motion (molecular dynamics) was strongly suppressed upon binding of HiP-8.
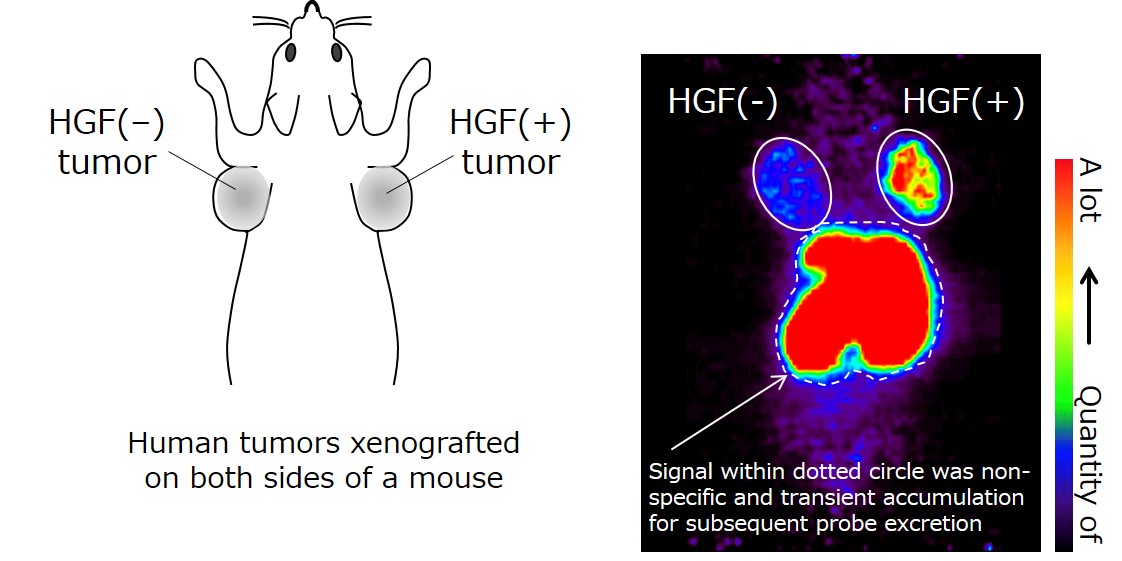
Figure 3. PET examination using HiP-8 as a molecular probe.
HiP-8 (probe) is observed to be accumulated in an HGF(+) tumor (xenografted to a mouse). The HiP-8 signal in the liver and kidney indicates the transient accumulation of HiP-8 for subsequent excretion. The cancer tissue in which HGF level was higher was visualized with a high contrast, compared to HGF(−) tumor.
[Glossary]
*1) Hepatocyte Growth Factor (HGF)
HGF, a physiologically active protein, discovered in Japan, plays important roles in regeneration in the liver and the kidney and in the protection of neurons. HGF not only stimulates proliferative activity of cells but enhances cell migration and survival. Excessive production of HGF in cancer tissue leads to cancer metastasis and resistance against anti-cancer agencies.
*2) Macrocyclic peptide
A peptide consists of several to dozens of amino acids chemically connected by peptide bond to form a chain-like structure. A macrocyclic peptide has a chemical bond between two constituent amino acid residues; hence it has a ring-like structure. Recently much attention has been paid to drug candidates having macrocyclic peptide structures.
*3) RaPID
Abbreviation of Random Peptide Integrated Discovery. RaPID system was established by Prof. Suga (University of Tokyo). This is a method to screen and obtain macrocyclic peptides rapidly and efficiently and select those which bind to target biological molecules (primarily proteins) with a high specificity and affinity. By using an artificial tRNA synthetic system (Flexizyme) and translation (from RNA to protein) system, an enormous number of different macrocyclic peptides are synthesized out of natural amino acids (20 types) as well as non-natural amino acids. From the various macrocyclic peptides, those which bind to a target molecule can be obtained with high efficiency.
*4) High-Speed Atomic Force Microscopy (HS-AFM)
HS-AFM was developed at Kanazawa University not only for academic but also for practical use. A very sharp probe attached to a flexible cantilever scans the surface of a specimen by flexibly changing the relative position between the probe and the specimen in horizontal directions. This enables visualization of the surface geometry and movement of the specimen.
*5) PET
PET is an acronym of Positron Emission Tomography. A chemical compound or a protein is labeled with a positron-emitting radioligand and the resultant labeled compound or protein, called a PET probe, is introduced into the body. The distribution or accumulation of PET probe in the body is visualized in 3-dimensions in a non-invasive manner and quantified. PET is highly effective in obtaining an image and time course of physiological functions in humans. By converting a drug into a PET probe, it becomes possible to investigate distribution of the drug among various organs. Imaging diagnosis is widely used using a PET probe that is known to accumulate in a specific diseased tissue.
Article
Macrocyclic peptide-based inhibition and imaging of hepatocyte growth factor
Journal: Nature Chemical Biology
Authors: Katsuya SAKAI1, Toby PASSIOURA2, Hiroki SATO1, Kenichiro ITO2, Hiroki FURUHASHI1, Masataka UMITSU3, Junichi TAKAGI3, Yukinari KATO4, Hidefumi MUKAI5, Shota WARASHINA5, Maki ZOUDA5, Yasuyoshi WATANABE5, Seiji YANO1, Mikihiro SHIBATA1, Hiroaki SUGA2, Kunio MATSUMOTO1
1. Kanazawa University, 2. The University of Tokyo, 3. Osaka University, 4. Tohoku University, 5. Riken
DOI: 10.1038/s41589-019-0285-7
Funder
This work was supported by World Premier International Research Center Initiative (WPI), MEXT, Japan. This work was supported in part by the A-STEP (Adaptable and Seamless Technology Transfer Program through Target-driven R&D) (grant no. AS262Z) from the Japan Science and Technology Agency (JST), the Medical Research Fund of Takeda Science Foundation, the Mitani Foundation for Research and Development, the Grant-in-Aid for JSPS Scientific Research (C) (no. 16K08544) to K.S., a Grant-in-Aid for JSPS Scientific Research (B) (no. 15K14473) to K.M., Project for Cancer Research and Therapeutic Evolution (P-CREATE) from the Japan Agency for Medical Research and development (AMED) to Y.W., H.M., K.M. and T.P., Basic Science and Platform Technology Program for Innovative Biological Medicine from AMED to H. Suga, a Grant-in-Aid for JSPS Research Activity Start-up (no. 16H06830) to H. Sato, a Grant-in-Aid for JSPS Fellows (no. 23-7727) to K.I., a Grant-in-Aid for JSPS Scientific Research (B) (no. 18K01836) to M.S. This work was performed under the Cooperative Research Program of the Institute for Protein Research, Osaka University (no. CR15-05) and an Extramural Collaborative Research Grant from the Cancer Research Institute (Kanazawa University).



 PAGE TOP
PAGE TOP