Abstract:
Electrochemical imaging reveals that nitrogen and phosphorus atoms are key for enhancing the ability of holey graphene to promote the release of hydrogen during electrolysis.
An international research group has improved graphene’s ability to catalyse the ‘hydrogen evolution reaction’, which releases hydrogen as a result of passing an electronic current through water. They designed a mathematically-predicted graphene electrocatalyst, and confirmed its performance using high resolution electrochemical microscopy and computational modelling. The findings were published in the journal Advanced Science.
Akichika Kumatani of Tohoku University’s Advanced Institute for Materials Research (AIMR), Tatsuhiko Ohto of Osaka University, Yoshikazu Ito of Tsukuba University, Yasufumi Takahashi of Kanazawa University and colleagues in Japan and Germany found that adding nitrogen and phosphorus ‘dopants’ around the well-defined edges of graphene holes enhanced its ability to electrocatalyse the hydrogen evolution reaction.
Graphene-based catalysts have an advantage over metal-based ones in that they are stable and controllable, making them suitable for use in fuel cells, energy storage and conversion devices, and in water electrolysis. Their properties can be improved by making multiple simultaneous changes to their structures. But scientists need to be able to ‘see’ these changes at the nanoscale in order to understand how they work together to promote catalysis.
Kumatani and his colleagues used the recently developed scanning electrochemical cell microscopy (SECCM) for direct, sub-microscale observation of the electrochemical reactions that happen when current is passed through water during electrolysis. It also allowed them to analyse how structural changes in graphene electrocatalysts affect their electrochemical activities. This type of observation is not possible using conventional approaches.
The team synthesized an electrocatalyst made from a graphene sheet full of mathematically predicted holes with well-defined edges. The edges around the holes increase the number of active sites available for chemical reactions to occur. They doped the graphene sheet by adding nitrogen and phosphorus atoms around hole edges. The graphene-based electrocatalyst was then used to enhance the release of hydrogen during electrolysis.
Using SECCM, the team found that their graphene electrocatalyst significantly improved the formation of a current in response to energy release during electrolysis. Their computational calculations suggest that adding nitrogen and phosphorus dopants enhances the contrast of positive and negative charges on the atoms surrounding hole edges, boosting their ability to transport an electric current.
Nitrogen- and phosphorus-doped holey graphene electrocatalysts worked better than those doped with only one of the two chemical elements.
“These findings pave a path for atomic-level engineering of the edge structure of graphene in graphene-based electrocatalysts through the local visualization of electrochemical activities,” the researchers conclude.
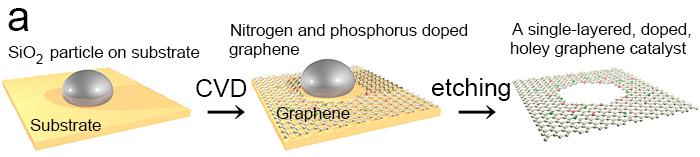
Figure 1.
Carbon atoms were deposited on a substrate using chemical vapour deposition. Silicon oxide nanoparticles on the substrate ensured the formation of holes. Nitrogen and phosphorus atoms were added. Ultimately a single-layered, doped, holey graphene catalyst was formed.
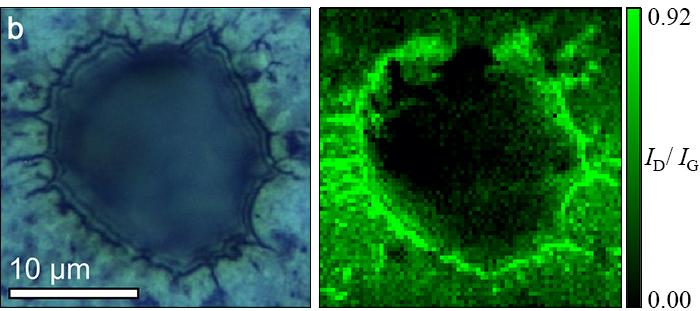
Figure 2. Optical image and Raman mapping (ID/IG) of an edge region.
Article
Chemical Dopants on Edge of Holey Graphene Accelerate Electrochemical Hydrogen Evolution Reaction
Journal: Advanced Science
Authors: Akichika Kumatani, Chiho Miura, Hirotaka Kuramochi, Tatsuhiko Ohto, Mitsuru Wakisaka, Yuki Nagata, Hiroki Ida, Yasufumi Takahashi, Kailong Hu, Samuel Jeong, Jun‐ichi Fujita, Tomokazu Matsue, Yoshikazu Ito
DOI: 10.1002/advs.201900119
Funder
JST-PRESTO “Creation of Innovative Core Technology for Manufacture and Use of Energy Carriers from Renewable Energy”; Grant-in-Aid for Scientific Research on Innovative Areas “Discrete Geometric Analysis for Materials Design”; JSPS KAKENHI; World Premier International Research Center Initiative (WPI), MEXT, Japan; NIMS microstructural characterization platform and Molecule & Material Synthesis Platform as a program of “Nanotechnology Platform Project,” MEXT, Japan; University of Tsukuba Basic Research Support Program Type S; the Iwatani Naoji Foundation; Intelligent Cosmos Academic Foundation



 PAGE TOP
PAGE TOP