Abstract:
We established a method for activity-dependent visualization of neural circuits in the fruit fly brain. An optogenetic method for reactivation of those visualized neural circuits upon illumination was also established for their manipulation. With these methods, we identified a neural cluster important for behavioral motivation. It is expected that these methods and findings would contribute to elucidation of the neural basis of innate behaviors of insects and regulation of their behaviors.
[Background]
Insects show a variety of species-specific innate behaviors (instinctive behaviors). For example, a worker honeybee that has found flower nectar exhibits 8-shape waggle-dances upon returning to its beehive. A male moth that has detected a sex pheromone flies around to look for a female counterpart. There remain a number of questions about how a variety of innate behaviors are generated by functions of neural circuits in the insect brain.
In order to obtain full pictures of neural circuits and their functions responsible for innate behaviors, it is necessary to reveal neural circuits that are activated when an innate behavior takes place. A method is also required that can control insect behavior by manipulating activities of neural circuits in an artificial manner.
[Outline of research results]
The present research group at Kanazawa University has been actively engaged in research on functions of neural circuits, focusing on genes whose expression occurs in a neural activity-dependent manner. Previously the group identified a transcription factor gene Hormone receptor 38 (Hr38) *1) that is expressed in a neural activity-dependent manner in the insect brain and found that this gene is a useful marker for neural activities.
In the present study, the group used the fruit fly (Drosophila melanogaster), a model insect, to generate a genetically modified strain that precisely reflects the expression pattern of Hr38, establishing a method that can specifically visualize active neurons by labeling them with green fluorescent protein (GFP)*2). Using this method, they revealed a full picture of the male fruit fly’s neural circuits in the brain and ventral nerve cord*3) that were activated when a male fly interacted with a female fly. It is known that the sex-determining gene fruitless and doublesex determines the sex of neural systems in the brain and ventral nerve cord of the fruit fly, and that these genes are responsible for development of male-type and female-type neural circuits. In the present study, the group applied their method specifically to neural circuits that express fruitless or doublesex. This revealed active neural circuits within the male-type neural circuits during the courtship behavior (Figure 1). As a result, the group found that a neural cluster aSP2 was active specifically when a male fly interacted with a female fly in addition to the neural circuits already known to be important in regulating the mating behavior.
In addition to the visualization of neural circuits activated during a behavior, it is important to be able to manipulate neural circuit activity in a desired manner to reveal neural circuit functions. Therefore, the group generated a Drosophila strain that can activity-dependently express CsChrismon, a light-activated channel rhodopsin*4), in place of GFP. A male fly of this strain was allowed to experience mating with a female fly; on the following day, after removal of the female fly, the male fly alone was illuminated. The male fly, although in the absence of any female fly, showed abdominal bending typical of copulation behavior (Figure 2). This indicates that the neural circuits that were activated during the mating the previous day can be reactivated by light one day later.
Furthermore, the group analyzed the functions of the neural cluster aSP2 on mating behavior. Detailed analysis of courtship behavior of the male flies whose aSP2 neural activity was inhibited revealed that male flies approached female flies in a normal manner but showed frequent interruption of courtship; as a result, mating success rate was much reduced. In the courtship of the fruit fly, the persistent dynamic approach of male flies to females, who may be reluctant at first, is important in the females’ acceptance of mating. The present result shows that the neural cluster aSP2 plays an important role in regulation of motivation during courtship behavior.
[Future prospects]
The present study has established for the first time methods to visualize neural circuits in an activity-dependent manner in insects and to manipulate their activity. These methods should be applicable to the elucidation of neural circuits and their functions in innate behaviors of insects. Understanding the neural basis of innate behaviors of insects is not only significant in fundamental science but could contribute to applications such as the efficient utilization of beneficial insects like the honeybee and the silkworm, getting rid of noxious insects, prevention of epidemics of diseases like malaria, dengue fever, Zika fever, etc. mediated by the mosquito. This study identified the neural cluster aSP2 as an important neural circuit for motivation of an insect behavior. It is expected that further research on the mechanism of how aSP2 neural circuit controls motivation will allow elucidation of fundamental regulatory mechanisms of innate behaviors in the insect brain.
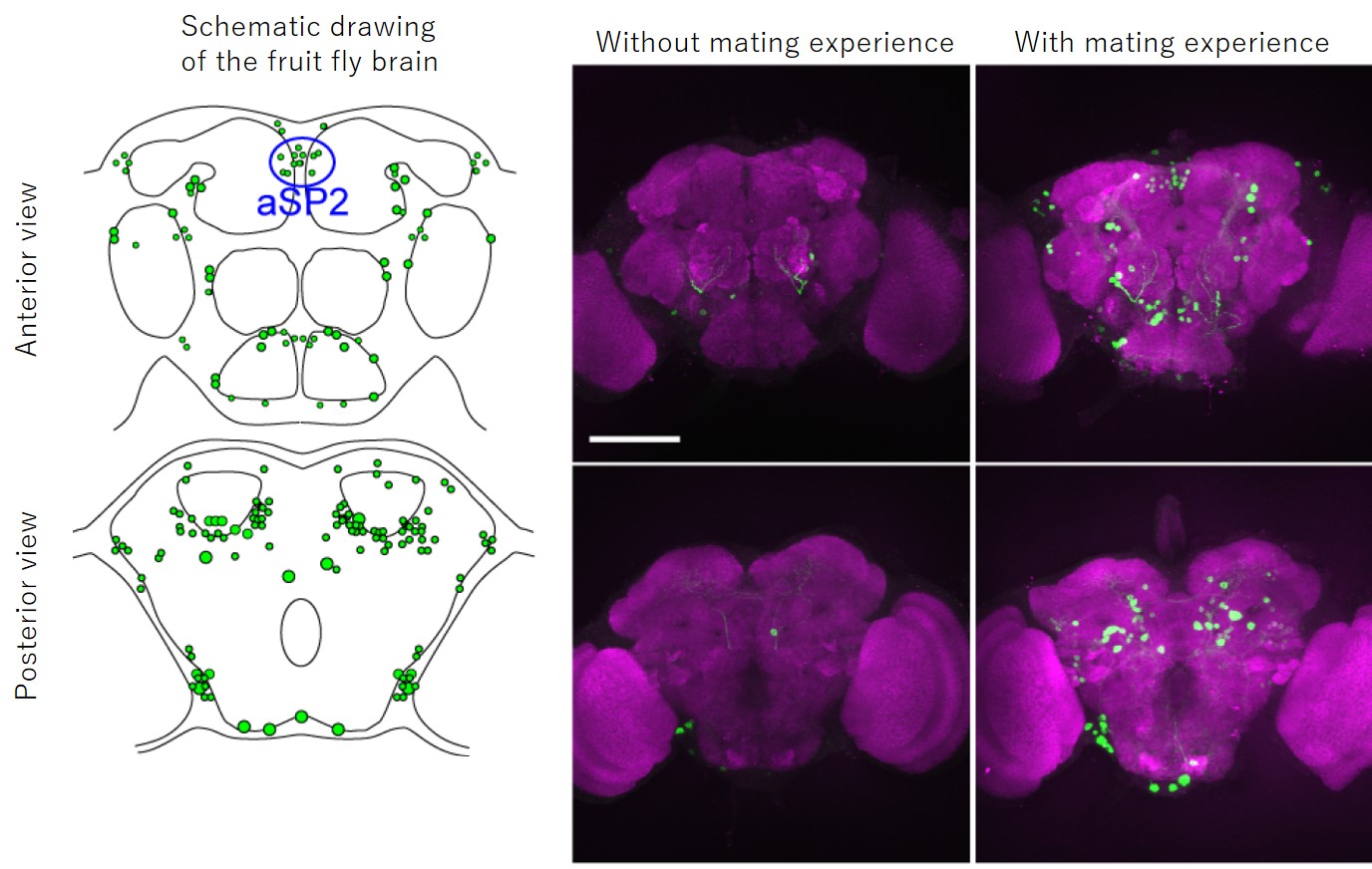
Figure 1. Activity-dependent visualization of fruitless neural circuits.
On the left are schematic drawings of the fruit fly brain. Neural cells, which express GFP upon mating behavior, are shown in green (upper drawing shows an anterior view, while the lower, a posterior view).
Right panels are pictures of the brains of male flies with or without mating experience. The entire brain is stained purple and the neural cells that were active during mating are stained green. Scale bar, 100 μm.
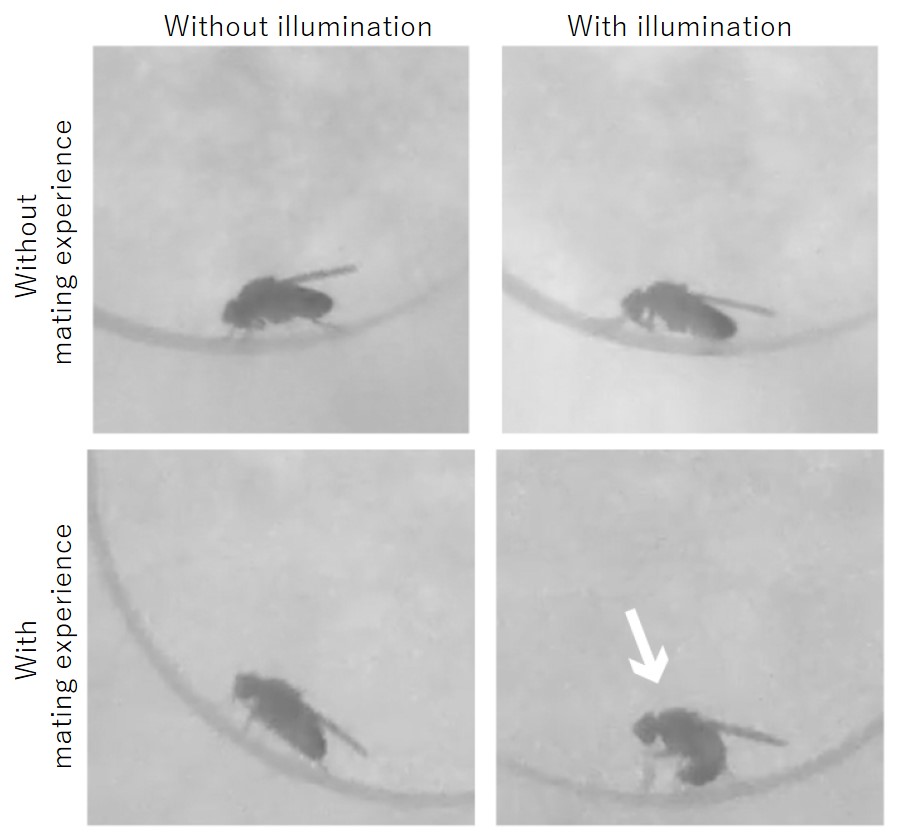
Figure 2. Optogenetic reactivation of male fruit flies’ neural circuits that express CsChrimson in an activity-dependent manner.
The male flies without mating experience did not show mating behavior upon illumination (upper photos), while the male flies with mating experience did show mating behavior, abdominal bending (indicated by an arrow), upon illumination.
[Glossary]
*1) Hormone receptor 38 (Hr38)
One of nuclear orphan receptors of insects. The present research group identified this gene that was expressed in a neural activity-dependent manner in the insect brain in 2013 and could be used as a marker gene for neural activities.
*2) Green fluorescent protein
Green fluorescent protein (GFP) was originally discovered in light-emitting jellyfish, Aequorea victoria by Dr. Osamu SHIMOMURA, a Nobel Laureate in Chemistry in 2008. When illuminated with blue light, it emits green fluorescence. GFP is widely used in visualization of neural cells, for example.
*3) Ventral nerve cord
Ventral nerve cord is the insect nerve region corresponding to the spinal cord of the vertebrates. It exists in the thorax in the fruit fly, consisting of neural cells for conveying sensory information to the brain and commands of the brain to muscles.
*4) Channel rhodopsin
Channel rhodopsin is a light-driven ion channel originally found in unicellular green algae, Chlamydomonas reinhardtii. The channel is regulated to be open/closed by light so when it is expressed in neural cells, it becomes possible to regulate neural activities by light.
Article
Activity-dependent visualization and control of neural circuits for courtship behavior in the fly Drosophila melanogaster
Journal: Proceedings of the National Academy of Sciences
Authors: Seika TAKAYANAGI-KIYA, Taketoshi KIYA
DOI: 10.1073/pnas.1814628116
Funder
Japan Society for the Promotion of Science (JSPS) KAKENHI Grants 26850218, 16H05053 and 18K14813, JSPS Research Fellow 201800091, Sasakawa Scientific Research Grants, Naito Foundation, Yamada Science Foundation, Hokuriku Bank, Takeda Science Foundation, Asahi Glass Foundation, Uehara Memorial Foundation, and Japan Prize Foundation.



 PAGE TOP
PAGE TOP