Abstract:
Currently, few antimalarial treatments exist that effectively kill liver-stage malaria parasite Plasmodium vivax, which can lay dormant for months or even years. Researchers at Kanazawa University have reported a new drug that could eliminate liver-stage malaria parasites completely. Using an insect virus, known as a baculovirus, the researchers investigated the ability of baculovirus to mediate innate immunity against malaria infection. This work could pave the way for developing new and more effective antimalarial treatments.
Kanazawa, Japan – Currently, few antimalarial treatments exist that effectively kill liver-stage malaria parasites, which can lay dormant for months or years as in the case of Plasmodium vivax. Researchers from Kanazawa University have successfully demonstrated that administration of a baculovirus virion (BV) completely eliminates liver-stage parasites in a mouse model via BV-induced fast-acting innate immunity. Further development of BV-based drugs could lead to newer and more effective treatments for malaria.
Malaria is caused by Plasmodium, a parasite spread by the Anopheles mosquito as it feasts on blood. The parasite is released into the bloodstream and travels to the liver to mature, before being released back into the bloodstream where it infects red blood cells. Symptoms normally appear a few days or weeks later, but in the case of P. vivax, the parasites can also lay dormant in the liver with disease recurring months or even years later (known as hypnozoites). P. vivax is the most widely distributed human malaria parasite in the world (a major health risk to 2.85 billion people worldwide). The active blood-borne form of P. vivax can be targeted with artemisinin, but only a single drug, primaquine, is available for the hypnozoites.
However, primaquine is associated with a high risk of life-threatening hemolytic anemia in people with glucose-6-phosphate-dehydrogenase enzyme deficiency. In addition, even effective doses can cause several side effects including nausea and vomiting. “Malarial infection affects a large number of individuals each year, many of whom are young children aged under five.” says first author Talha Bin Emran. “Current treatments can have serious side effects for some individuals, hence safer radical curative drugs that efficiently kill the hypnozoites are urgently needed.”
Using BV, the researchers conducted a series of experiments with a mouse model of malaria. They confirmed that intramuscular administration of BV not only provides complete protection against a subsequent sporozoite infection but also eliminates existing liver-stage parasites completely, which could prevent or reduce the severity and complications of the disease. The elimination of liver-stage parasites with BV was superior to that with primaquine. Additionally, they showed that the elimination effect occurred in a TLR9-independent manner. These effects were mainly mediated by a cytokine known as interferon alpha (IFN-α), which has previously been investigated as a treatment for several other diseases.
Further work is needed to confirm the results in primates and eventually humans, but initial results suggest that there are several potential benefits of BV as a new non-haemolytic single-dose alternative to primaquine. “Currently P. vivax patients must receive several doses of antimalarials for treatment, therefore adding BV to existing drugs could reduce the risk of infection whilst receiving treatment.” study corresponding author Shigeto Yoshida says. “It could also provide protection against the disease in the liver. There are several challenges in the treatment of malaria, which we hope to overcome with our work.” These results demonstrate the potential to develop new malaria drugs that kill P. vivax hypnozoites over an extended period and with reduced side effects.
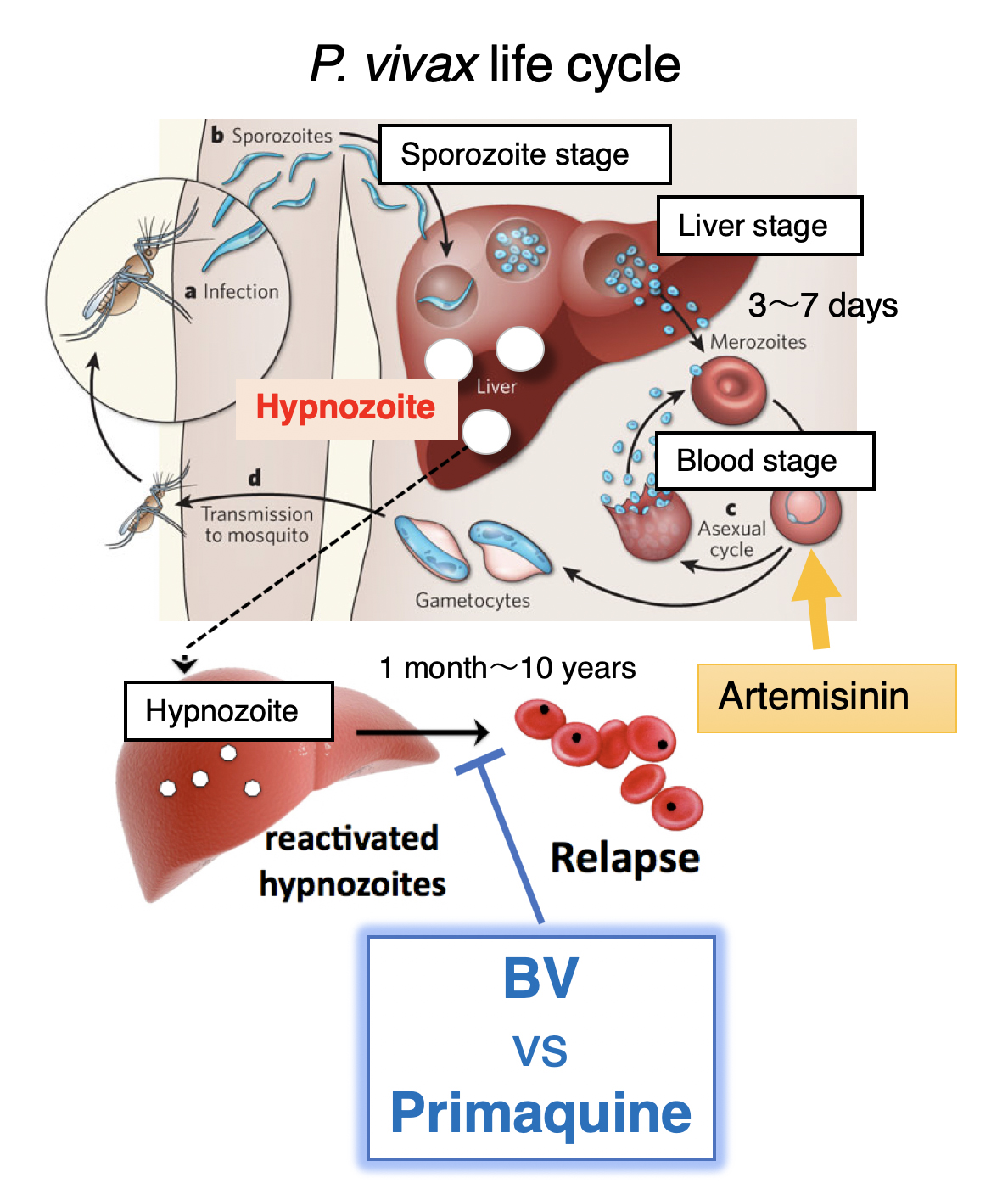
Figure 1. P. vivax life cycle
A proportion of P. vivax sporozoites differentiate to a hypnozoite form that ultimately reactivates and proliferates leading to a blood-stage relapse. The hypnozoites are not eradicated by artemisinin and can awaken months to years after the last bout of clinical malaria, unless a drug specifically targetting the hypnozoite, primaquine, is administered. However, primaquine has severe side effects.
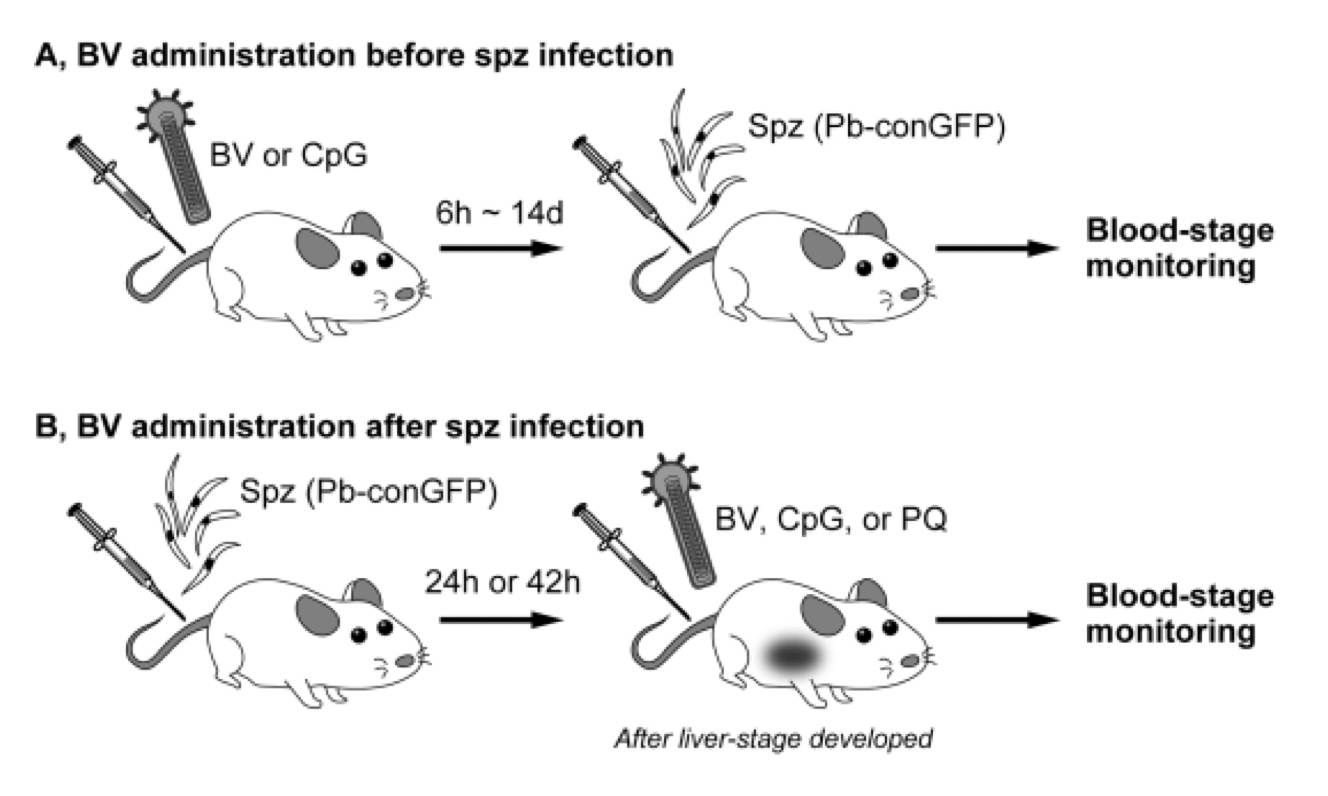
Figure 2. Experimental design
(A) Mice were intramuscularly administered BV or CpG followed by an intravenous challenge with malaria sporozoites (Pb-conGFP) at various time intervals (6 h–14 d). A group of mice administered BV were protected. (B) Mice were intravenously injected with Pb-conGFP sporozoites. At 24 after liver-stage development, the mice were administrated with either BV, CpG, or primaquine. Blood-stage parasites were monitored after sporozoite injection. Once parasites appeared in the blood, all mice died. (Results) BV administration before and after sporozoite infection sterilely protected mice, while all mice administrated with CpG or primaquine were died.
Article
Baculovirus-Induced Fast-Acting Innate Immunity Kills Liver-Stage Plasmodium
Journal: Journal of Immunology
Authors: Talha Bin Emran, Mitsuhiro Iyori, Yuki Ono, Fitri Amelia, Yenni Yusuf, Ashekul Islam, Asrar Alam, Megumi Tamura, Ryohei Ogawa, Hiroyuki Matsuoka, Daisuke S. Yamamoto and Shigeto Yoshida
DOI: 10.4049/jimmunol.1800908
Funders
This work was supported by a Grant-in-Aid for Young Scientists (B) (Japanese Society for the Promotion of Science [JSPS] KAKENHI Grant 26860278), the Japan Foundation for Pediatric Research (2015), and cooperative research grants from the Nagasaki University Institute of Tropical Medicine (NEKKEN 2014-7 Grants 26-6, 27-5, 28-6, and 29-3) (to M.I.) and by Grants-in-Aid for Scientific Research (B) (JSPS KAKENHI Grant 21390126 and 25305007) and a Grant-in-Aid for Challenging Exploratory Research (JSPS KAKENHI Grant 24659460) (to S.Y.). T.B.E. was supported by Ministry of Education, Culture, Sports, Science, and Technology Fellowship 153343.



 PAGE TOP
PAGE TOP