Abstract:
Gut microbiota-derived metabolites play important roles in health and disease. In this study, we show the pathophysiological role of D-serine in association with the gut microbiota in humans and mice with acute kidney injury. The results demonstrate the renoprotective effects of D-serine derived from the gut microbiota, shed light on the interactions between the gut microbiota and the kidney, and highlight D-serine as a potential new therapeutic target and biomarker for acute kidney injury.
[Background]
The kidney is an organ not only for excreting body waste by urine but also for maintaining body homeostasis in close cooperation with other organs. By producing various hormones, the kidney, for example, generates new erythrocytes in the bone marrow through erythropoietin, maintain bones turnover through activation of vitamin D, and controls blood pressure through renin-angiotensin system. In addition, it has recently been shown that the kidney plays an important role in affecting longevity. However, the relationship of the kidney to the gut (the gut microbiota*1)) have not been studied in detail.
Amino acids exist as enantiomers, i.e., D-amino acids and their L-forms. The term “amino acids” has generally referred to the L-amino acid form or to the mixture of the L- and D- enantiomers. Recent progress in analytical technologies now makes it possible to distinguish the D-amino acids and their L-forms of about 20 amino acid species that are essential in our body. These amino acids are referred to as chiral amino acids. The D-amino acids and their L-forms of an amino acid have the same chemical formula, but are mirror images in three-dimensional structure, analogous to the difference between the left and right hand. Most interestingly, the D-amino acids and their L-forms differ significantly in their functions in living systems. L-amino acids are constituents of proteins, one of the most important components of living cells. On the other hand, the functions and mechanisms of production of D-amino acids are less clear.
[Results]
Researchers from Kanazawa University, in collaboration with those from Waseda University, RIKEN, Okayama University, Kyushu University and Kitasato University have investigated kidney functions in relation to the gut (the gut microbiota), paying particular attention to the fact that the kidney maintains body homeostasis and the internal environment in cooperation with a number of other organs.
To assess the effect of acute kidney injury (AKI)*2) on gut microbiota, we performed gut microbiota analysis with mouse feces after ischemia/reperfusion (I/R) injury to an AKI model.
The gut microbiota were examined to reveal that specific gut bacteria were influenced by AKI (Fig. 1). In addition, we explored the contribution of the gut microbiota to the pathogenesis of AKI. I/R injury was induced in germ-free (Gf) mice that have no gut microbiota with or without fecal transplantation from normal mice. I/R injury was worse in the Gf B6 mice than in the normal B6 mice. Interestingly, fecal transplantation from normal mice attenuated the renal pathology in the Gf B6 mice. These results suggested that the gut microbiota changed due to I/R injury and that possible substance(s) protective for the kidney was produced by the gut microbiota.
Next, in order to identify the substance(s) protecting the kidney against I/R injury produced by gut microbiota, comprehensive analyses of chiral amino acids were performed. While various D-amino acids were detected in the feces, only D-serine was detected in the kidney (Fig. 2a). The result suggested that D-serine was produced by the gut microbiota of AKI mice and transported to the kidney via the blood circulation (Fig. 2b). Furthermore, since D-serine was not detected in the feces of Gf mice, it was suggested that gut microbiota produced D-serine in response to AKI. In addition, D-serine metabolizing enzymes in the kidney were found to increase the D-serine concentration after I/R injury. Thus, the D-serine concentration in the kidney increased due to augmentation of D-serine production in the kidney in addition to the production of D-serine by the gut microbiota after I/R injury.
Further, in order to examine the effects of D-serine produced by the gut microbiota on the kidney, D-serine dissolved in drinking water was administered to normal mice. The oral administration of D-serine mitigated the kidney injury in normal mice and D-serine-depleted mice. These results showed that D-serine played roles in protecting the kidney from AKI.
Lastly, it was investigated whether similar mechanisms exist in human patients with AKI. The blood D-serine level of such patients was found to be higher than that of healthy subjects, showing a high correlation with creatinine, one of the markers of renal disorders.
Thus, it was revealed that some gut bacteria respond to AKI, producing D-serine, a substance protective for the kidney, thus affecting the kidney via the blood circulation (Fig. 3).
[Future prospects]
This study has elucidated the mechanism by which the kidney interacts with gut microbiota through the D-amino acid. Further, genomic information concerning the gut microbiota that change upon AKI has been deposited in DDBJ/GenBank/EMBL from the present study. Information on the chiral amino acid analyses has also been published. These are expected to contribute to further studies on the kidney and the gut microbiota and to more studies of chiral amino acids.
In the future, it will be necessary to establish whether the change of D-amino acid levels due to AKI appear more rapidly than the biomarkers*3) known so far, and whether analogous mechanisms exist for chronic renal disorders.
We expect that the present study will contribute to the development of biomarkers and medications for AKI based on the utilization of D-amino acids.
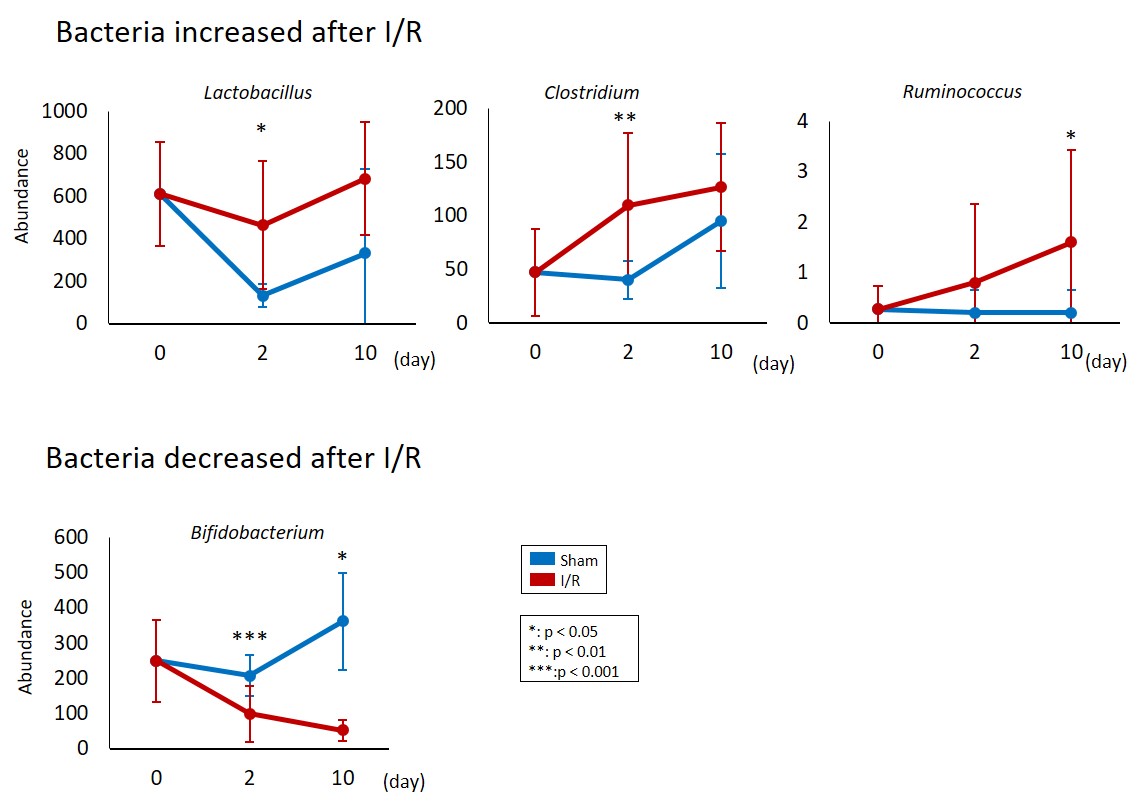
Figure 1. AKI-induced gut dysbiosis in the mouse I/R model.
Comparison of the abundance of several species between the I/R-injured and the sham-operated mice at pre-I/R and days 2 and 10.
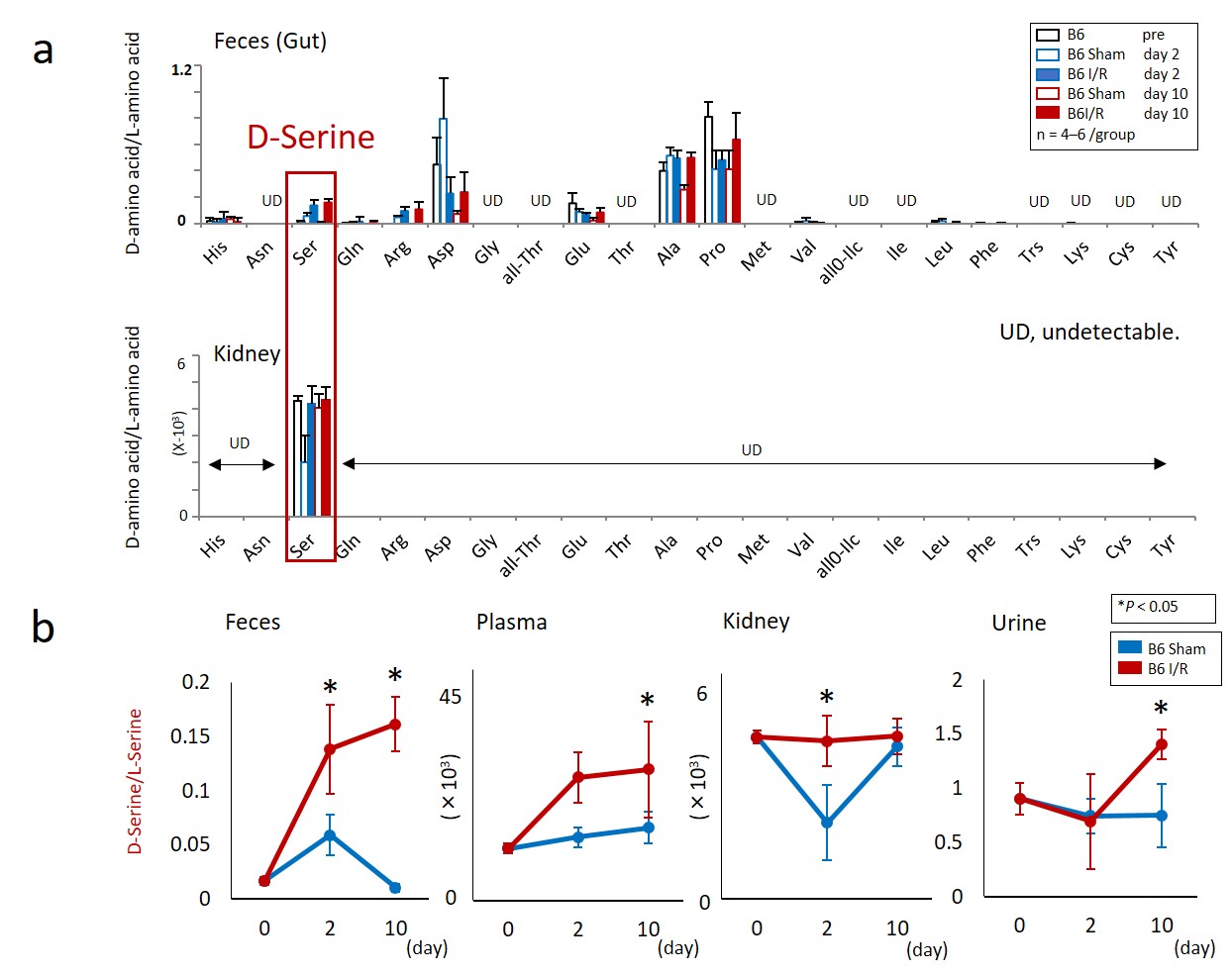
Figure 2. Dysbiosis induced by AKI alters the balance of D/L-amino acids.
(a) Although some free D-amino acids were detected in the feces of B6 mice with or without I/R, only D-serine can be detected in the kidney. (b) Free D-serine is increased in the feces, plasma, kidney, and urine after the I/R.
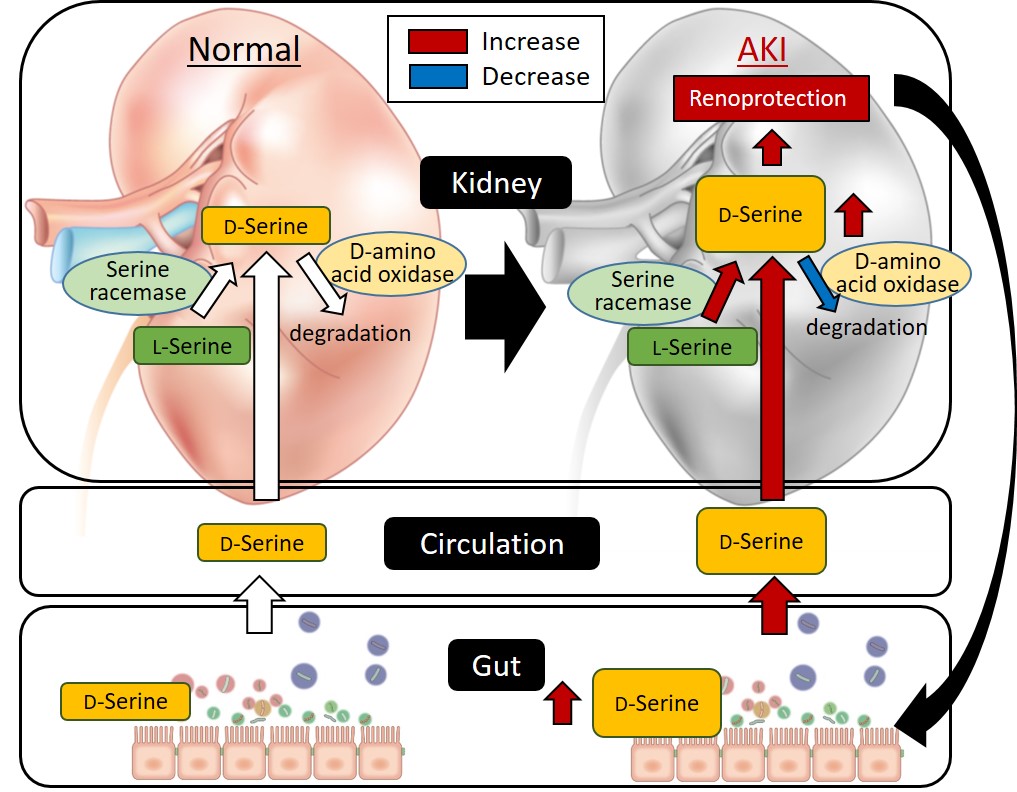
Figure 3.
Proposed model of the relationship between gut-derived D-serine and the kidney based on the results of this study.
[Glossary]
*1) Gut microbiota
The gut microbiota is the complex community of microorganisms that live in the digestive tract. Those microorganisms, mainly bacteria, live not separately but inter-dependently, being influenced by and influencing the host bodies. Healthy human subjects are thought to have a stable population of gut bacteria. On the other hand, human subjects with disease are thought to have altered gut microbiota that influence the whole body in various ways.
*2) Acute kidney injury
Renal diseases can be divided into two forms, acute and chronic. Acute kidney injury induction refers to surgical treatment to induce an acute renal disorder in an artificial manner. Mouse models that resemble human acute renal disorders can be generated by pinching the blood vessel to the mouse kidney with a clip for a period of time, which prevents blood flow into the kidney, and then by re-opening the blood vessel upon removing the clip.
*3) Biomarker
A biomarker is a measurable indicator of a biological state or condition. Biomarkers are evaluated using blood, for example. With renal diseases, creatinine and eGFR are known to be useful biomarkers reflecting renal functions.
Article
Gut microbiota-derived D-serine protects against acute kidney injury
Journal: The Journal of Clinical Investigation Insight
Authors: Yusuke NAKADE, Yasunori IWATA, Kengo FURUICHI, Norihiko SAKAI,Takashi WADA, and 23 others
DOI: 10.1172/jci.insight.97957
Funders
Japan Society for the Promotion of Science KAKENHI grant



 PAGE TOP
PAGE TOP