Abstract:
Researchers from Kanazawa University developed a three-dimensional open-loop electric potential microscopy technique to visualize the charge accumulation behavior at the interface between a solid electrode and liquid electrolyte. The technique was used for providing information about the charge distribution at the interface between a copper wire electrode and salt-based electrolyte. This technique increases our ability to probe nanoscale interfacial phenomena, making it useful for research in electronics, electrochemistry, and biology.
Kanazawa, Japan—Charges and their transport are integral to the function of electronic devices, batteries, and biological systems. The charges that accumulate at the interface between a solid electrode and an electrolytic solution containing ions that carry charges, can affect the electrode-electrolyte interaction as well as processes such as corrosion and molecular adhesion. Consequently, it is important to obtain a clear picture of accumulated charges at such interfaces to improve our understanding of interfacial phenomena in a variety of systems. However, imaging the three-dimensional (3D) spatial distribution of accumulated charges at interfaces has been difficult because it is challenging to measure the lateral charge distribution at a solid–liquid interface.
A team based at Kanazawa University has developed a microscopy approach called 3D open-loop electric potential microscopy (OL-EPM) to visualize the real-space charge distribution at the interface between an electrode and electrolyte. The researchers developed 3D OL-EPM by first optimizing their existing two-dimensional OL-EPM technique.
“Conventional OL-EPM is limited by influence of the long-range interaction between the sample and microscope tip and cantilever,” says the first author Kaito Hirata. “We minimized this influence by improving the equations used to calculate the potential in OL-EPM.”
These improved equations enabled to subtract the long-range force acting on the microscope tip and cantilever from the measured data. As a result, the short-range forces originating from charges accumulated in the electric double layer were observed as changes of the local surface potential. The ability of the improved equations to calculate interfacial charge distributions was determined using two electrodes with different charge accumulation behavior. The opposite charge accumulation characteristics at the two electrodes were successfully captured using the improved OL-EPM equations.
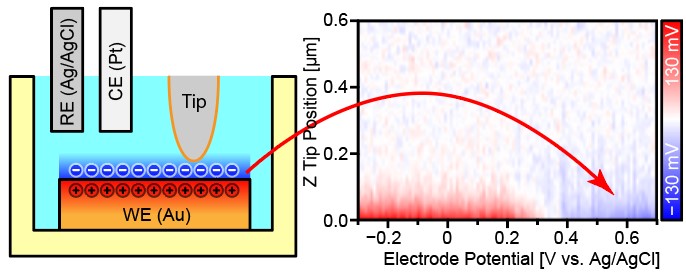
The improved OL-EPM approach was then combined with a 3D tip scanning technique to provide 3D OL-EPM. The team used 3D OL-EPM to visualize the charge accumulation at the interface between a copper wire electrode and salt electrolyte. The obtained results provided valuable information about the charge distribution at the electrode–electrolyte interface.
“We can use 3D OL-EPM to investigate electrochemical reactions and local solution conditions at electrode–electrolyte interfaces,” explains the corresponding author Takeshi Fukuma. “The information obtained from such experiments is important for fields such as electrochemistry, electronics, and biology.”
The ability to obtain real-space data about the nanoscale charge distribution at electroactive interfaces promises to increase our understanding of interfacial phenomena and aid progress in electronics and battery research.
Figure
Charge accumulation behavior at the Au-electrolyte interface was visualized by three-dimensional open-loop electric potential microscopy with varying electrode potential.
Article
Visualizing charges accumulated in an electric double layer by three-dimensional open-loop electric potential microscopy
Journal: Nanoscale
Authors: Kaito Hirata, Takuya Kitagawa, Keisuke Miyazawa, Takahiro Okamoto, Akira Fukunaga, Chikako Takatoh and Takeshi Fukuma
DOI: 10.1039/c8nr03600d
Funders
This work was supported by ACT-C, the Japan Science and Technology Agency and the World Premier International Research Center Initiative (WPI), MEXT, Japan.



 PAGE TOP
PAGE TOP