Abstract:
A Japanese research team led by Kanazawa University analyzed the structure of six natural products from an Australian shrub, Cryptocarya laevigata. The compounds contained a spiro[3.5]nonane moiety—cyclohexene sharing a single carbon with cyclobutane—never previously seen in nature, as well as a lactone. The cyclobutane ring is possibly biosynthesized from two dissimilar alkenes, which is rare. The absolute configuration (chirality) remains unclear, but the team is working on synthesis to allow biological activity testing.
Kanazawa – The botanical world can be an exciting place for chemists. Plant species produce a beautiful array of organic molecules with complex structures, often of great practical use. Indeed, this is a realm where new discoveries are still being made. Recently, a Japanese-led research team discovered an entirely new structural class in compounds from a jungle-dwelling shrub.
The glossy or red-fruited laurel (binomial name: Cryptocarya laevigata) inhabits the rainforests of eastern Australia. Little was known about the chemical makeup of this tall shrub until the team, led by Kanazawa University, analyzed an extract of its twigs and leaves. The plant’s essential oil was found to contain a family of six new compounds, the structural analysis of which revealed some surprises.
As reported in Organic Letters, NMR experiments showed that at the center of the compounds lay a peculiar, nine-membered carbon cycle known as a spiro-nonene. This structure consists of two rings of carbon atoms—one containing six atoms, the other four—linked by a single “pinch point” atom that is a part of both rings. This motif had never been seen before in any natural product.
Four-carbon rings—cyclobutanes—are rare because of their unstable structure. Carbon generally prefers to build larger rings, which are less strained. A few cyclobutanes do exist in nature; however, the Kanazawa team thinks that C. laevigata cyclizes these particular compounds in a unique way, reacting a monoterpene with a polyketide, instead of two identical alkenes as is usual.
The intricacy doesn’t stop there. The C. laevigata products also contain lactone groups—cyclic esters—prompting the name of these compounds, “cryptolaevilactones.” Esters are often associated with fruity aromas and perfumery. Three of the compounds in fact contain bicyclic lactones, where the ester is cyclized into two interlocking rings. However, as study co-author Fumika Tsurumi admits, “These might be artifacts of the purification process. Further experiments will help to clarify this.”
“Nature is often the most inventive organic chemist,” adds lead author Kyoko Nakagawa-Goto. “In our lab we’re still working on synthesizing these cryptolaevilactones from scratch. In fact, we have yet to fully establish the chirality. The absolute configuration between the cyclobutane and lactone is unclear. Once we have produced larger amounts, which are not available from the plant extracts, we can test them for useful biological activity.”
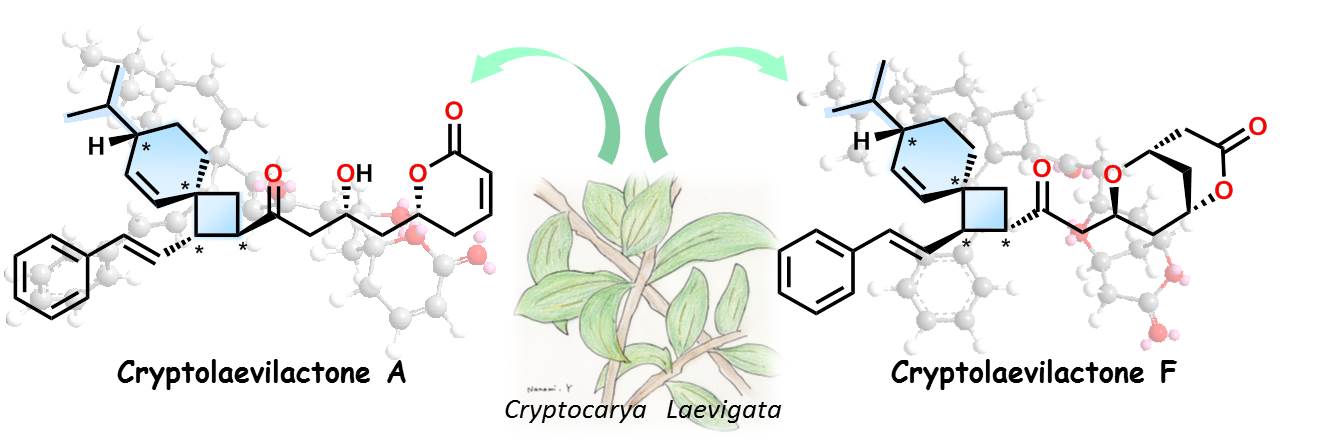
Figure 1.
Isolations of unique monoterpene-polyketides with spiro[3,5]nonane from C. laevigata.
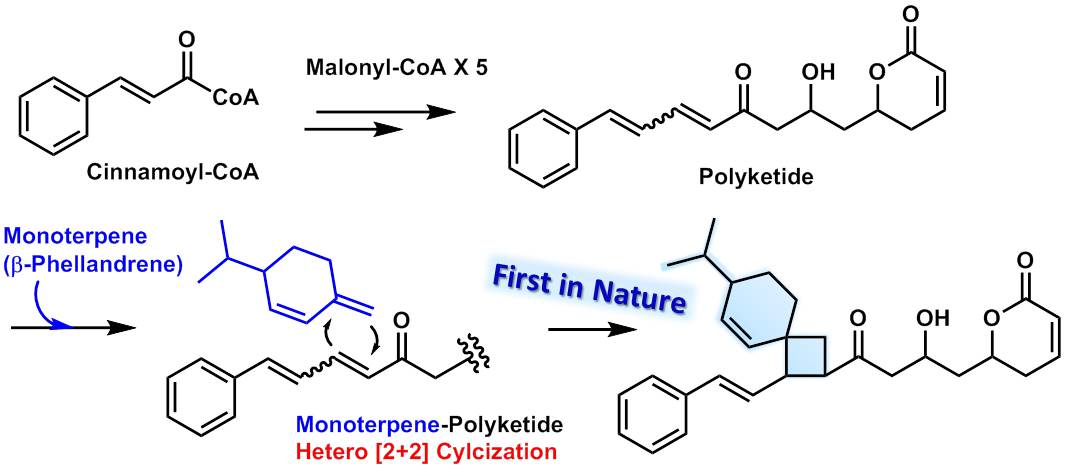
Figure 2.
Proposed biosynthetic pathway of cryptolaevilactones
Article
Secondary Metabolites, Monoterpene–Polyketides Containing a Spiro[3.5]nonane from Cryptocarya laevigata
Journal: Organic Letters
Authors: Fumika Tsurumi, Yuta Miura, Yohei Saito, Katsunori Miyake, Tetsuo Fujie, David J. Newman, Barry R. O’Keefe, Kuo-Hsiung Lee, and Kyoko Nakagawa-Goto
Doi: 10.1021/acs.orglett.8b00624.
Funders
This work was supported by JSPS KAKENHI (Grant No. 25293024, awarded to K.N.G.). Partial support is also acknowledged from NIH Grant CA177584 awarded to K.H.L.



 PAGE TOP
PAGE TOP