Abstract:
An excessively unbalanced diet, especially high-fat diet, is one of the causes of lifestyle diseases and cancer. Here, we find that in mouse hematopoietic stem cells, Spred1 suppresses high-fat diet-induced ERK signaling activation to prevent leukemogenesis. It is also found that in Spred1-deficient mice, high-fat diet-induced leukemogenesis is partly mediated by alterations to the gut microbiota, suggesting that abnormal gut microbiota may participate in leukemogenesis. Our results may contribute to leukemogenesis prevention and treatment improvement.
[Background]
Hematopoietic stem cells (HSCs) *1) can give rise to all types of blood cells during whole life of animals. HSCs possess multipotency to supply all types of blood cells as well as to reproduce themselves (self-renewal) in order not to be depleted. Since the body is always exposed to various kinds of stresses such as inflammation and reactive oxygen species, it is considered that a system is necessary to protect HSCs from various stresses in order to continuously supply all types of blood cells throughout life.
Recently, it has been known that an excessively unbalanced diet, especially a high-fat diet, may be one of the important factors to cause various diseases including lifestyle related diseases and cancer. However, it was not known how stresses caused by an excessively unbalanced diet would affect HSCs.
[Results]
The present team of Kanazawa University, Japan, together with researchers of domestic and foreign institutions has been conducting research on molecules and signaling pathways involved in HSC self-renewal. The team found that the ability for self-renewal of HSCs was regulated by ROCK signaling*2) controlled by the Spred1*3) molecule. Furthermore, the team discovered that Spred1 in HSCs was activated by stimuli like cytokines*4), aging, radiation damage, bacterial infection and so on. Here, roles of Spred1 in HSCs were investigated by using Spred1-deficient mice. Under normal conditions, Spred1-deficient mice did not show any significant disorder of the blood; however, under a condition with one of the stressors mentioned above, while wildtype mice showed damage of HSCs, Spred1-deficient mice showed either reduced damage or augmentation of HSC functions (Figure1). These results suggest that although Spred1 functions to suppress self-renewal of HSCs, it does not play important roles in HSCs’ giving rise to the blood cells under normal conditions.
Next, by carrying out experiments under various forms of stress, the team has found that Spred1-deficient mice initiate leukemogenesis and die after having continuously been fed a high-fat diet containing large amounts of lard. With these Spred1-deficient mice, HSCs have been found to lose normal functions but their ERK signaling*5) has been aberrantly activated to initiate leukemogenesis. These results indicate that Spred1 suppresses activation of tumorigenesis-stimulating signaling pathways induced by excessive fat intake and that Spred1 plays an important role in maintaining normal HSC functions against excessive nutritional stress.
In addition, they investigated the reasons why excessive fat intake initiates leukemogenesis in the Spred1-deficient mice. It has been found that the ratio of gram-positive bacteria*6) in the gut is increased in the mice fed a high-fat diet in comparison with the mice fed a normal diet, equilibrium of the gut microbiota*7) being disrupted. By administering antibiotics to the Spread1-deficient mice fed a high-fat diet to eliminate bacteria in the gut, suppression of reduction of HSC functions is observed along with suppression of initiation of leukemogenesis. With these results, the research team concludes that a high-fat diet affects regulation of HSCs by altering the gut microbiota. Under normal conditions, Spred1 protects HSCs from losing their activity against dietary stress-induced initiation of leukemogenesis, whereas a failure of the system damages HSCs, possibly initiating leukemogenesis (Figure 2).
[Future prospects]
A well-balanced and appropriate diet is one of the most important factors for maintaining good health. The present research using mice indicates that, even with continuous excessive fat meals, Spred1 plays an important role as a safeguard of HSCs under normal conditions to avoid serious abnormalities of the blood. Recent research has, however, revealed that some leukemia patients have low expression levels of Spred1 and that such patients suffer from severer pathology. Therefore, if Spred1 function is impaired for some reason, continuous overeating and overdrinking would possibly lead to abnormality of the blood. The team hopes to elucidate the pathophysiology of blood abnormalities such as leukemia, by investigating the regulation of expression level of Spred1 and gut microbiota-derived factors induced by excessively unbalanced dietary habits, and by further investigating regulatory mechanisms of Spred1 functions. It is hoped further research will lead to prevention of leukemia and to improvement of therapy.
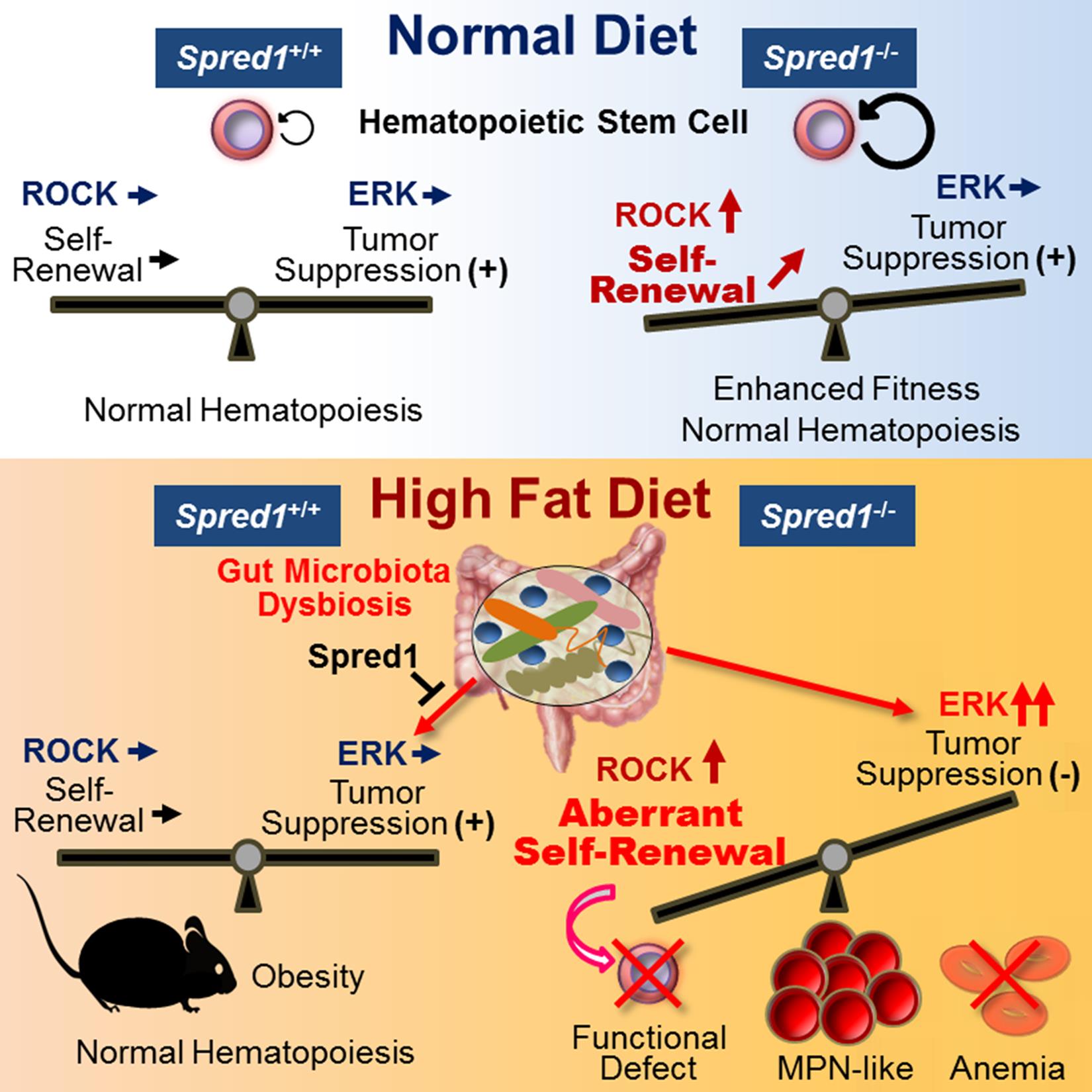
Figure. Graphical abstract of the paper
The wildtype HSCs of mice fed a normal diet give rise to ordinary blood cells, having regulation of HSC self-renewal by ROCK signaling balanced with suppression of ERK signaling by cancer-suppressing functions. On the other hand, Spred1-deficient HSCs exhibit augmented ROCK signaling, which accentuates self-renewal ability and stress resistance. Nonetheless, cancer-suppressing functions are maintained to a moderate degree, so even Spred1-deficient HSCs do not initiate leukemogenesis, giving rise to normal blood cells.
Mice fed a high-fat diet exhibit alterations to the gut microbiota. Mice having wildtype HSCs become obese because of the presence of Spred1 but their blood remains normal. On the other hand, if Spred1 is deficient, HSCs show aberrant self-renewal due to ROCK signaling activation and due to de-repression of ERK signaling. As a consequence, Spred1-deficient HSCs cannot be normal, losing stem cell functions, which results in severe anemia and in initiation of leukemogenesis.
[Glossary]
*1) Hematopoietic stem cell
The cell giving rise to all types of blood cells throughout life. It also possesses ability for self-renewal in order not to deplete the hematopoietic stem cells themselves.
*2) ROCK signaling
Rock signaling regulates abilities of cell-cell adhesion and cell migration by regulating cell morphology. In cancer cells, it plays roles in cancer cell invasion and metastasis.
*3) Spred1
Causative gene of human Legius syndrome. Spred1 plays roles to suppress cytokine.mediated effects in the cell.
*4) Cytokines
Cytokines are a group of proteins secreted by cells, inducing various reactions of cells such as cell proliferation, cell differentiation and apoptosis of cells in proximity of the cells that secrete cytokines. Cytokines affect the cells that secrete cytokines themselves.
*5) ERK signaling
ERK signaling upregulates cell proliferation. In many cancers, upregulation of ERK signaling is detected. In general, aberrant regulation of ERK signaling is considered to be one of the causes of tumorigenesis.
*6) Gram-positive bacteria
Gram-positive bacteria are bacteria that give a positive result in the Gram stain test; they occur in the gut microbiota. Some types of gram-positive bacteria have been reported to produce substances damaging cells of the host animal given a high-fat diet.
*7) Gut microbiota
As soon as a baby is born, a variety of bacteria settle in his/her digestive tract. The whole bacterial community in the gut is referred to as the gut microbiota. Recent research has revealed that alterations to the gut microbiota affect our health and are involved in various diseases.
Article
Spred1 Safeguards Hematopoietic Homeostasis against Diet-Induced Systemic Stress
Journal: Cell Stem Cell
Authors: Yuko Tadokoro, Takayuki Hoshii, Satoshi Yamazaki, Koji Eto, Hideo Ema, Masahiko Kobayashi, Masaya Ueno, Kumiko Ohta, Yuriko Arai, Eiji Hara, Kenichi Harada, Masanobu Oshima, Hiroko Oshima, Fumio Arai, Akihiko Yoshimura, Hiromitsu Nakauchi, and Atsushi Hirao
Doi: 10.1016/j.stem.2018.04.002
Funders
Japan Society for the Promotion of Science (JSPS), The Ministry of Education, Culture, Sports, Science and Technology (MEXT), World Premier International Research Center Initiative (WPI) from MEXT, Japan Agency for Medical Research and Development (AMED), and Bristol-Myers Squibb



 PAGE TOP
PAGE TOP