Abstract:
Researchers at Kanazawa University report in EMBO Reports a new molecular mechanisms regulating cellular fate of squamous cell carcinomas
[Background]
Squamous cell carcinoma (SCC)*1 is a lethal cancers arising from the stratified epithelia of skin, esophagus, cervix, as well as the head and neck tissues. Genomic analysis of SCCs identified genomic amplification of TP63 in up to 30% of tumors, with overexpression of its mRNA in the majority of SCCs.
ΔNp63α, one of the proteins encoded by TP63, has an important role in the epithelial development and maintenance. In SCCs, DNp63a functions as a key transcriptional regulator of different gene subsets in order to maintain or enhance malignant phenotypes. However, the mechanism controlling the nuclear transport of this protein, were, up to now, unclear.
Nucleoporins (NUPs) are a family of proteins building nuclear pore complexes (NPC)*2 and mediating nuclear transport across the nuclear envelope. Recent evidence suggests a cell-type-specific function for certain NUPs; however, the significance of NUPs in SCC biology remains unknown.
[Results]
In the present study, Hazawa et al. show that one particular nucleoporin, nucleoporin 62 (NUP62), is highly expressed in stratified squamous epithelia, and is further elevated in SCCs. They further demonstrate that depletion of NUP62 inhibits proliferation and augments differentiation of SCC cells, suggesting NUP62 is required for preventing epidermal differentiation of SCCs. The impaired ability to maintain the undifferentiated status is associated with defects in ΔNp63α nuclear transport. Finally, they unmasked the detailed traffic machinery where the pro-differentiation Rho kinase (an enzyme that catalyzes the transfer of phosphate groups) inhibits the nuclear transport of ΔNp63α by reducing the interaction between NUP62 and ΔNp63α.
[Future prospects]
This study demonstrates the role of NUP62 regulating cellular fate of SCCs through ΔNp63α nuclear transport. However, whether these NUPs regulates cell identity in different tissues (or in other types of cancer cells) is still an open question. As the authors comment in the paper: “Our finding of convertible trafficking activity of NUP62 highlights the potential for therapeutic targeting of nuclear transport of this oncogene.”
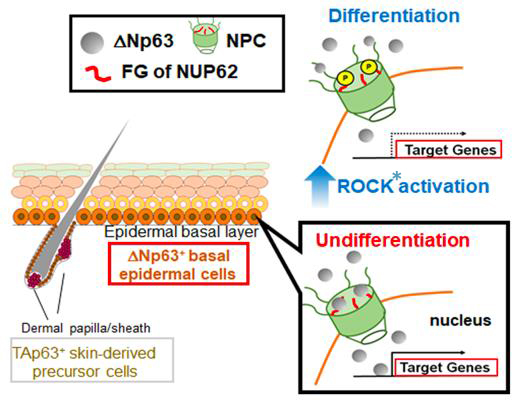
Figure: Hypothetical model of NUP62 action in regulating cell fate.
* Rho-associated protein kinase (ROCK)
A kinase (that is, an enzyme that catalyzes the transfer of phosphate groups) involved in regulating the shape and movement of cells by acting on the cytoskeleton.
[Glossary]
*1 Squamous cell carcinoma (SCC)
SCC is the cancerous growth of cells in the squamous cells, which include the cells in the upper layers of the skin (the epidermis). SCC is mainly caused by excessive exposure to ultraviolet light over the course of many years. SCCs may affect different part of the body, including the skin, the esophagus and the cervix, and is particularly common in the head and neck.
*2 Nuclear pore complex (NPC)
The nucleus of eukaryotic cells is surrounded by a double membrane separating it from the cytoplasm, the thick solution that fills cells. Nuclear pore complexes are large protein complexes that cross this membrane, allowing the transport of molecules. The proteins that make up the nuclear pore complex are called nucleoporins.
Article
Title: ROCK‐dependent phosphorylation of NUP62 regulates p63 nuclear transport and squamous cell carcinoma proliferation
Journal: EMBO Reports
Authors: Masaharu HAZAWA, De-Chen LIN, Akiko KOBAYASHI, Yan-Yi JIANG, Liang XU, Firli Rahmah Primula DEWI, Mahmoud Shaaban MOHAMED, Hartono, Mitsutoshi NAKADA, Makiko MEGURO-HORIKE, Shin-ichi HORIKE, H. Phillip KOEFFLER, Richard W. WONG
Doi: 10.15252/embr.201744523
Funders
Japan Society for the Promotion of Science (JSPS) the Grants-in-Aid for Scientific Research, World Premier International Research Center Initiative (WPI), MEXT, Japan, and Institute for Frontier Science Initiative, Kanazawa University



 PAGE TOP
PAGE TOP