Abstract:
We have developed high-speed frequency modulation AFM (FM-AFM) and enabled atomic-resolution imaging in liquid at ∼1 s/frame. With this AFM, we have obtained images revealing the transition region is formed along the step edges. Our simulations suggest the transition region be a Ca(OH)2 monolayer formed as an intermediate state. Thus, our understanding of the calcite dissolution in water is much improved. This FM-AFM will be applicable to various studies on solid−liquid interface dynamic processes.
[Background]
Calcite is one of the most abundant components of the Earth crust, the outer-most layer of the Earth, constituting as the largest carbon reservoir in the global carbon cycle in nature. Thus, large-scale dissolution of calcite would give an enormous impact on the weather, geography, aquatic environment and so on; more specifically, for example, changes in the carbon dioxide concentration of the air and the acidity of the ocean. Recently, the dissolution mechamism of calcite attracts much attention because of its importance in geologic carbon sequestration (GCS) technology to capture carbon dioxide from the air and to store it underground. In order to precisely predict such a large-scale and long-term phenomenon, the dissolution mechanism of calcite should be understood at an atomic level in a precise manner.
When a crystal of calcite is immersed in water (Figure 1a), it is observed that a monolayer of ~0.3 nm thickness (height), called the step*, is formed on the surface exposed to water and that the crystal dissolution proceeds as desorption of atoms from the step edege to aqueous solution. Therefore, understanding of atomistic events at step edges is essential for elucidation of the dissolution processes. Nonetheless, due to the limitations of measurement technologies, it was difficult to directly observe high-speed structural changes associated with the atomistic dissolution process. Thus, many aspects of crystal growth and dissolution mechanism, including those of calcite, remained unclear.
AFM has a truly unique advantage, that is, capability of observation of the surface morphology of insulating materials. Therefore, AFM is thought to be a measurement technique that may have great potential to solve the problem described above. Nonetheless, conventional AFMs, even the leading-edge ones, did not have enough spatial or temporal resolution, which did not allow the problem to be solved nor the atomistic movements to be observed.
[Results]
The researchers of Kanazawa University, Japan, have been leading the development of technologies for frequency modulation AFM (FM-AFM) over years, and have revolutionized the temporal resolution to be ~1 s/frame from the conventional one, ~1 min/frame. The international research team led by the researchers of Kanazawa University, with Aalto University, Finland and Tohoku University, Japan, succeeded in direct observation of the dissolution processes of calcite surface in water as well as of structural changes around step edges at atomistic level for the first time in the world. Moreover, from the FM-AFM images, the team has found that the transition region of a few nm width along a step is formed as an intermediate state in the dissolution processes (Figure 1b). The formation of this transition region was not foreseen by previous studies, so that without our present high-speed FM-AFM, it would not have been discovered.
In addition, in order to elucidate the origin of the transition region and dissolution mechanism, the team examined the validity of various transition region models by density functional theory calculations and by molecular dynamics simulations (Figure 2). It was found that the transition region would most likely be a Ca(OH)2 monolayer formed as an intermediate state in the dissolution processes of calcite. Based on these results, the team proposes a dissolution mechanism at atomistic level as follows (Figure 3).
1. At the step edges, dissociative adsorption of a water molecule leads to generation of ion pair of surface-bound CaOH+ and free HCO3–.
2. HCO3– is decomposed and surface-bound Ca(OH)2 and free CO2 are formed.
3. Repetition of these reactions forms the transition region consisting of a Ca(OH)2 monolayer at the step edge.
4. At the peripheries of the transition region, stability of the surface-adsorbed Ca(OH)2 molecules depends on the distance from the step edge, and at a certain distance (typically, a few nm) or more, Ca(OH)2 dissociates.
To the team’s knowledge, this is the very first proposal for the dissolution processes at atomistic level based on such direct experimental evidences. Moreover, this is also the first proposal for the dissolution mechanism of calcite with the formation of transition region taken into consideration. Thus, the team believes that the present study promotes the understanding of calcite dissolution mechanism at atomistic level to a great extent.
[Significance and future development]
The precise understanding of the dissolution processes of calcite at atomistic level obtained in this study may enable us to comprehend the physical meanings of empirical parameters used for simulations of the dissolution processes at a macroscopic level. This may also lead to accurate prediction of dissolution behaviors in various solution environments in nature, and the present study is expected to make contributions, in the future, to better prediction accuracy of the global carbon cycle. Furthermore, the high-speed FM-AFM developed and reported in this study will be applicable not only to studies of the dissolution processes of calcite but to those of crystal growth, dissolution and self-assembly of a variety of minerals and organic as well as biological molecules. It will also be quite useful for observation and investigation of a wide variety of solid-liquid interface phenomena at atomistic level such as metal corrosion, catalytic reaction, etc. Since there were no appropriate direct observation means available for those phenomena, the present high-speed FM-AFM is expected to pave the way for discoveries of various phenomena so far unknown.
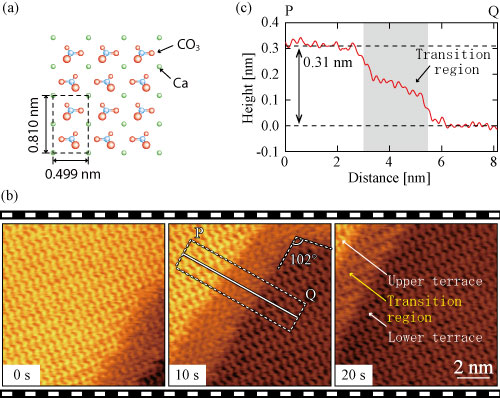
Figure 1: (a) Atomistic model of calcite surface. (b) The dissolution processes of calcite surface in water observed with high-speed FM-AFM. It is observed that the step is moving from lower-right to upper-left. Along the step is also seen the transition region. (c) Averaged height profile measured along the line PQ indicated in (b). The height of a monolayer step is ~0.3 nm, but that of the transition region is smaller. A terrace described in the Figure indicates a flat area at the atomic level on the crystal surface. The upper terrace is higher by one monolayer of CaCO3 than the lower terrace.
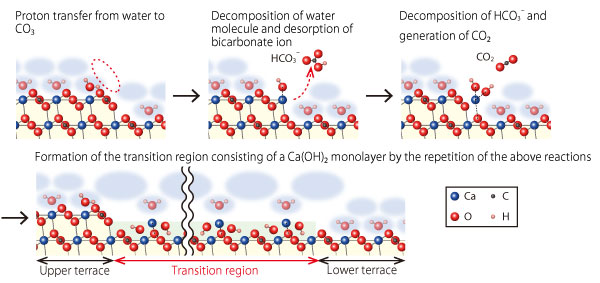
Figure 2: A simulation model of the transition region.
With a model that places a monolayer of Ca(OH)2 in proximity of a step at the boundary of upper terrace and lower terrace, molecular dynamics simulation was performed for about 7.5 ns to confirm that the monolayer of Ca(OH)2 existed stably adjacent to the step without being separated from the crystal surface.
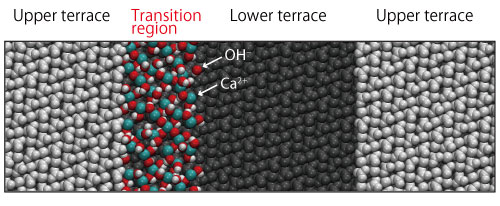
Figure 3: Atomistic dissolution model of calcite surface in water.
[Glossary]
*Step
On crystal surface, there are flat regions, called terrace, but at the periphery of a terrace, there are steps between the terraces having different heights. A step with a height of single layer of crystal-constituting molecules is called mono-molecular step.
Article
Title: Dissolution Processes at Step Edges of Calcite in Water Investigated by High-Speed Frequency Modulation Atomic Force Microscopy and Simulation
Journal: Nano Letters 2017
Authors:Kazuki MIYATA1, John TRACY2, Keisuke MIYAZAWA1, Ville HAAPASILTA2, Peter SPIJKER2, Yuta KAWAGOE1, Adam S. FOSTER1,2, Katsuo TSUKAMOTO3, Takeshi FUKUMA1
1Kanazawa University, Japan, 2Aalto University, Finland, 3Tohoku University, Japan
Doi: 10.1021/acs.nanolett.7b00757
Funder
KAKENHI and Bilateral Joint Research Project from JSPS, JST ACT-C, Kanazawa University CHOZEN Project, Centres of Excellence Program from the Academy of Finland, EU project PAMS.



 PAGE TOP
PAGE TOP