Abstract:
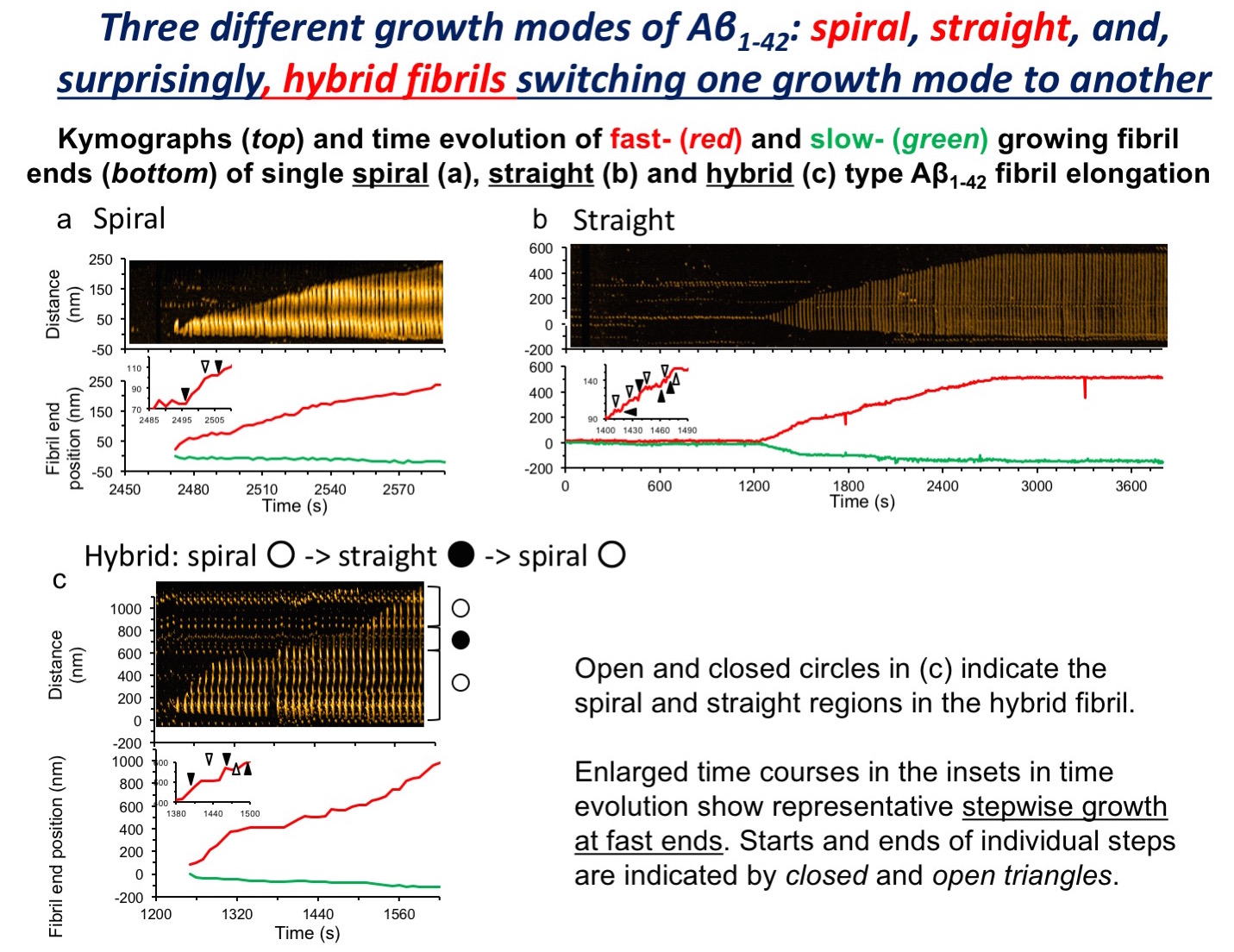
In the brain of patients with Alzheimer’s disease (AD), senile plaques are found, mainly consisting of amyloid β protein (Aβ) fibrils. Aggregation of Aβ1-42 to form fibrils plays a central role in AD pathogenesis. By utilizing high-speed AFM developed at Kanazawa University, we succeeded in, for the first time, video-imaging of Aβ1-42 fibril formation, and discovered switching between two fibril structures, “straight” and “spiral”. Also, the switching phenomenon was influenced by buffer salt composition, i.e., environmental conditions.
In the brain of patients with Alzheimer’s disease (AD), characteristic structures called senile plaques are found. The major component of senile plaques is amyloid fibrils consisting of amyloid β protein (Aβ). Processes of aggregation of Ab, particularly a molecular species of Ab1-42, have been considered to play a central role in the pathogenesis of AD. In addition, different structures of Ab aggregates are considered to be related to the difference in the disease phenotype of AD.
The research team of Kanazawa University in collaboration with UCLA succeeded in recording video images of the processes of Aβ1-42 aggregation by utilizing the world fastest atomic force microscopy (AFM) developed at Kanazawa University. The detailed analyses of the processes resulted in the following findings. While aggregation of Aβ gives rise to two types of fibrils, “straight” and “spiral,” one type of fibril transforms into the other type in the process of fibril formation; the switching is influenced by buffer salt composition, i.e., environmental conditions.
The research results obtained concerning the dynamic transformation, switching, of the aggregation structures of Aβ1-42 and its susceptibility to environmental conditions are expected to lead to novel disease-modifying methods for medical treatments and prevention of AD.
Article
Title: High-speed atomic force microscopy reveals structural dynamics of amyloid β1–42 aggregates
Journal: Proc. Natl. Acad. Sci. U.S.A.
Authors: Takahiro WATANABE-NAKAYAMA1, Kenjiro ONO1,2, Masahiro ITAMI1, Ryoichi TAKAHASHI1, David B. TEPLOW3, Masahito YAMADA1
1Kanazawa University, 2Showa University School of Medicine, 3University of California Los Angeles, U.S.A.
Doi: 10.1073/pnas.1524807113
Funder
Scientific Research (C) (26461266) and Grants-in-Aid (20390242) and (15K15336) from JSPS; Novartis Foundation; Takeda Science Foundation; NIH grant AG041295; a Grant from MHLW; SENSHIN Medical Research Foundation.



 PAGE TOP
PAGE TOP