Abstract:
The team led by researchers of Kanazawa University reports pathological changes in the brain of patients with Creutzfeldt-Jakob disease developing after cadaveric dura mater grafting. Significant association of cadaveric dura mater grafting with subpial amyloid β protein deposition and meningeal amyloid angiopathy, characteristics of Alzheimer’s disease, is demonstrated, which suggests that the amyloid β protein pathology propagated from the superficial portions of the brain in the patients with dura mater graft-associated Creutzfeldt-Jakob disease.
The human brain is covered with three layers of membranes called the meninges, the outermost layer of which is the dura mater. Between 1973 and 1997, a number of cadaveric dura mater grafting, a kind of transplant, were performed; in 1980s, it is estimated that 20,000 cases of such grafting were done in Japan per year. However, there were a number of cases where patients, after grafting, developed Creutzfeldt-Jakob disease (CJD), a rapidly progressing and fatal disease caused by infectious prion protein (PrPSc). Such CJD is considered to have developed due to PrPSc of the dura mater grafted.
The senile plaque, the structure specific to the brains with Alzheimer’s disease (AD), contains amyloid β protein (Aβ) as the main component. The process of aggregation and accumulation of Aβ is thought to play central roles in progression of lesion of brain with AD.
Recently, the possibility of human-to-human transmission of cerebral β-amyloidosis has also been suggested in autopsy studies of patients with iatrogenic CJD. In the present study, in order to evaluate the possibility of transmission of cerebral β-amyloidosis in humans via cadaveric dura mater graft, immunohistochemical studies were performed on patients with dura mater graft-associated CJD (dCJD) and other patients with sporadic CJD (sCJD) using antibodies against prion protein and Aβ.
Although total Aβ load did not differ significantly between patients with dCJD and sCJD, subpial Aβ deposition score in dCJD was significantly higher than that in sCJD. For the cerebral amyloid angiopathy (CAA) score, total CAA scores and meningeal CAA scores in dCJD were significantly higher than those in sCJD, but there were no significant differences in parenchymal CAA score or capillary CAA score.
In addition, in the patients with dCJD, the incubation period between dura mater grafting and death was significantly correlated with subpial Aβ deposition score, total CAA score and meningeal CAA score, although it was not significantly correlated with Aβ load, parenchymal CAA score or capillary CAA score.
The current results demonstrate significant association of cadaveric dura mater grafting with subpial Aβ deposition and meningeal amyloid angiopathy, suggesting that the Aβ pathology propagated from the superficial portions of the brain in the patients with dCJD. Cadaveric dura mater grafts were placed on the cerebral cortices, so cerebral β-amyloidosis might have spread directly to the superficial portions of the cerebral cortices.
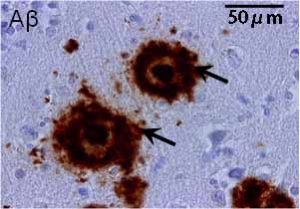
Senile plaques (indicated with arrows) with accumulation of amyloid β protein (Aβ) dyed in brown.
Article
Title: Significant association of cadaveric dura mater grafting with subpial Aβ deposition and meningeal amyloid angiopathy
Journal: Acta Neuropathologica
Authors: Tsuyoshi HAMAGUCHI1, Yu TANIGUCHI1, Kenji SAKAI1, Tetsuyuki KITAMOTO2, Masaki TAKAO3,4,5, Shigeo MURAYAMA3, Yasushi IWASAKI6, Mari YOSHIDA6, Hiroshi SHIMIZU7, Akiyoshi KAKITA7, Hitoshi TAKAHASHI7, Hiroyoshi SUZUKI8, Hironobu NAIKI9, Nobuo SANJO10, Hidehiro MIZUSAWA10,11, Masahito YAMADA1
1Kanazawa University, 2Tohoku University, 3Tokyo Metropolitan Institute of Gerontology, 4Mihara Memorial Hospital, 5Saitama International Medical Center, 6Aichi Medical University, 7Niigata University, 8National Hospital Organization Sendai Medical Center, 9University of Fukui, 10Tokyo Medical and Dental University, 11National Center of Neurology and Psychiatry
Doi: 10.1007/s00401-016-1588-3
Funder
a Grant-in-Aid from the Research Committee of Prion Disease and Slow Virus Infection; a Grant-in-Aid from the Research Committee of Surveillance and Infection Control of Prion Disease, MHLW; a Grant-in-Aid from the Research Committee of Molecular Pathogenesis and Therapies for Prion Disease and Slow Virus Infection, AMED; a Grant-in-Aid from JSPS (26430060); a Grant-in-Aid from Scientific Research on Innovative Area (Comprehensive Brain Science Network) from MEXT.



 PAGE TOP
PAGE TOP