Researchers at Kanazawa University report in STAR Protocols procedural details and tips for nanoendoscopy-AFM, for capturing images of nanoscale structures inside living cells.
————————————————————————————————————————————————————————————————
Images of nanoscale structures inside living cells are in increasing demand for insights into cellular structure and function they can reveal. So far, the tools for capturing such images have been limited in various ways, but researchers led by Takeshi Fukuma and Takehiko Ichikawa at Kanazawa University have now devised and reported a full protocol for using atomic force microscopy (AFM) to image inside living cells.
AFM was first developed in the 1980s and uses the changes in the forces between a sample surface and a nanoscale tip attached to a cantilever to “feel” surfaces and produce images of the topography with nanoscale resolution. The technique has grown increasingly sophisticated for extracting information about samples and at speeds sufficient for the tool to capture moving images of dynamics at the nanoscale. However, so far, it has been limited to surfaces. Other techniques exist that can provide a view of the inside of a cell but with limitations. For instance, there is electron microscopy, which is capable of resolving details at the nanoscale and smaller, but the required operating conditions are not compatible with living cells. Alternatively, fluorescence microscopy is regularly used on living cells, but while fluorescence techniques exist to increase the resolution, there are practical challenges that inhibit fluorescence imaging at the nanoscale. AFM suffers from neither limitation and by embellishing the tool with a nanoneedle to penetrate cells, Fukuma, Ichikawa and their collaborators have recently demonstrated the capability to image inside cells at the nanoscale, which they describe as nanoendoscopy-AFM.
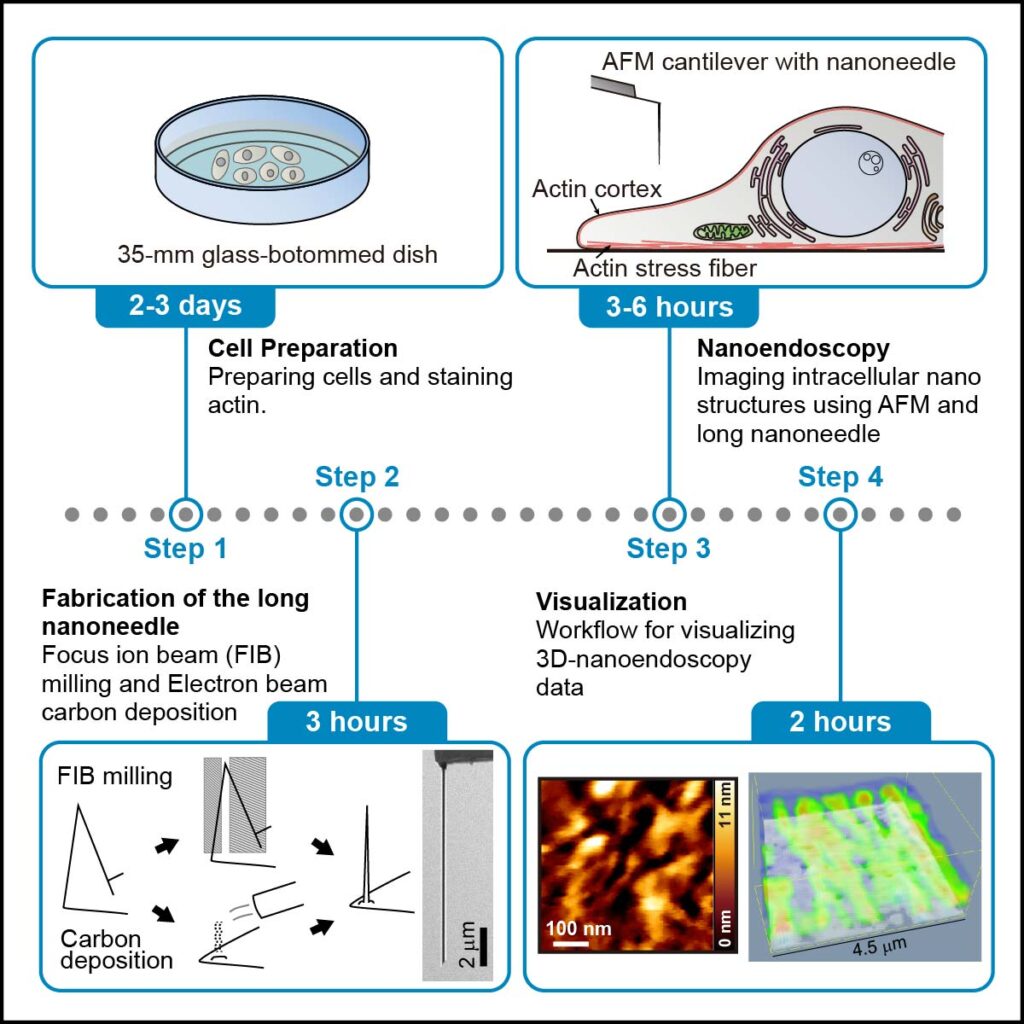
In their protocol, the researchers break down the method for nanoendoscopy-AFM into 4 stages. The first few steps involve cell preparation and staining with a fluorescent dye and checking the fluorescence, which is used to identify the imaging area quickly. Next is the fabrication of the nanoneedles themselves, for which there are two options – either etching away a nanoneedle structure with a focused ion beam or building one up with electron beam deposition. Then comes the nanoendoscopy stage itself, and in the report, the researchers describe the approach for both 2D and 3D nanoendoscopy. There are even details outlined to describe the best way to clean up after the nanoendoscopy images are captured before finally outlining the data processing needed to visualize the measured data. The method is replete with tips for successfully accomplishing each stage, as well as a guide for troubleshooting when things are not quite working out.
This technique should be suitable for the observation of intact intracellular structures, including mitochondria, focal adhesions, endoplasmic reticulum, lysosomes, Golgi apparatus, organelle connections, and liquid-liquid phase-separated structures. They conclude, “This protocol can be expected to become a standard tool for studying nanoscale structures.”
Glossary
Atomic force microscopy
Atomic force microscopy detects the attractive forces between a sample surface and tip from the resulting deflection of a cantilever that bears a nanoscale tip. Because the tip gradually tapers to such a small area, and the attractive forces dwindle so rapidly with distance, the topography of the surface can be obtained with nanoscale resolution. The technique was first reported shortly after the scanning conductance microscope, and although it was not the first to report an imaging resolution potential on the atomic scale, it had the great advantage of operation requirements that are compatible with non-conducting samples and living cells. It has also since provided valuable insights into dynamic insights with the development of a high-speed version of the tool at Kanazawa University. The development of this nanoendoscopy-AFM to penetrate cells extends the scope of the tool even further.
In one variation of AFM, the tip oscillates up and down as it is scanned over a structure, and the effect of changes in the resonance frequency of the oscillating tip gives an indication of the forces it is subject to. Tapping, dynamic, or AC AFM, as this has been described, has been attractive for imaging fragile structures as no contact is made. Here it is used inside the cell for 2D nanoendoscopy. An alternative is 3D nanoendoscopy, where at each point that is raster scanned, the tip is retracted by a specified distance, which may be between 0.1 and 1 micrometres. The measured force until it reaches the set point is then noted at each point.
Figure
Overview of the method for observing actin fibers in living cells using nanoendoscopy-AFM.
©2023 Ichikawa, et al., STAR Protocols
Reference
Takehiko Ichikawa, Mohammad Shahidul Alam, Marcos Penedo, Kyosuke Matsumoto, Sou Fujita, Keisuke Miyazawa, Hirotoshi Furusho, Kazuki Miyata, Chikashi Nakamura and Takeshi Fukuma. Protocol for live imaging of intracellular nanoscale structures using atomic force microscopy with nanoneedle probes. STAR Protocols 4, 102468 (2023).
DOI:10.1016/j.xpro.2023.102468
URL: https://doi.org/10.1016/j.xpro.2023.102468



 PAGE TOP
PAGE TOP