Abstract: Researchers at Kanazawa University elucidate in Frontiers in Molecular Biosciences how small biocontainers enclosed by membranes are involved in a disease called ATTRv amyloidosis.
Amyloidosis is the collective name for a group of diseases characterized by the deposition of amyloids — insoluble proteins that form due to the misfolding and aggregation of soluble proteins — outside of cells. Such depositions lead to cellular dysfunctions, and take place in patients with Alzheimer’s disease, Parkinson’s disease and dementia. In the disease called hereditary (variant) transthyretin amyloidosis (abbreviated ATTRv amyloidosis), variants of the transthyretin (TTR) gene lead to TTR amyloid deposits in several organs, with symptoms including muscle weakness and cardiac failure. It is known that the removal of amyloid proteins is promoted by so-called extracellular vesicles (EVs) — small ‘biocontainers’ enclosed by a membrane — but what is unclear is whether EVs are involved in the formation and subsequent deposition of TTR amyloids in the context of ATTRv amyloidosis. Rikinari Hanayama and colleagues from Kanazawa University have now studied the relationship between ATTRv amyloidosis and EVs, and confirm that the latter play an important role in the aggregation and deposition of TTR amyloids.
The researchers first analyzed the serum of ATTRv amyloidosis patients for traces of TTR amyloid. (Serum is blood without the clotting factors.) They found that TTR is present in EVs derived from serum, and that the so-called V30M mutation variant of TTR aggregates at the membranes of serum-derived EVs.
Hanayama and colleagues then looked at what happened when V30M-TTR amyloids were added to cell cultures, with and without serum-derived EVs. They found that V30M-TTR amyloid aggregates are deposited on cells in a much more pronounced way when serum-derived EVs are present, indicating that serum-derived EVs promote the aggregation of V30M-TTR and their deposition on cells.
From a comparison between ATTRv amyloidosis patients and healthy individuals, the scientists found that ATTRv amyloidosis is associated with a lower amount of TTR aggregates in serum-derived EVs. The hypothesis that emerges from the experiments is that in ATTRv amyloidosis patients, the presence of V30M-TTR and EVs leads to a self-enhancing uptake of EVs; this then leads to an enhanced deposition of TTR aggregates in tissue, resulting in a decrease of TTR aggregates in serum.
The findings of Hanayama and colleagues suggest that TTR in serum-derived EVs is a potential target for both ATTRv amyloidosis diagnosis and therapy. The researchers also point to the relevance of their results on our understanding of Alzheimer’s disease because TTR inhibits the nucleation of amyloid-β aggregation and its aggregation is promoted in EVs. In this respect, they conclude that “it is necessary to further analyze the relationship between TTR and […] EVs derived from the brain, in addition to EVs derived from the liver and other blood sources”.
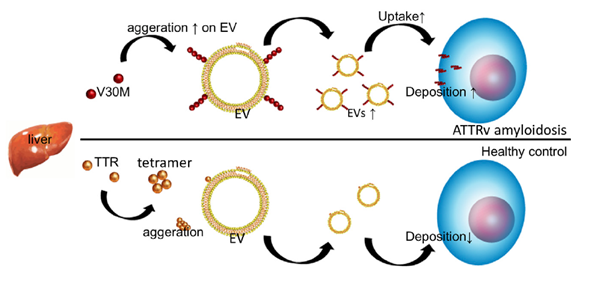
Figure 1.
Model of EV-mediated V30M-TTR cell deposition in ATTRv amyloidosis patients, compared to processes in healthy controls.
[Background]
Amyloidosis
Amyloidosis is a group of diseases in which abnormal proteins are deposited, and lead to build-ups, in tissue. Signs and symptoms associated with amyloidosis include fatigue, weight loss, shortness of breath, palpitations, and feeling faint when standing.
There are many different types of amyloidosis, each of which is due to a specific protein misfolding; the irregularity of these proteins can be due to genetic effects, as well as environmental factors.
In hereditary (variant) transthyretin amyloidosis (ATTRv amyloidosis), the protein that misfolds and aggregates is transthyretin (TTR). Depositions of the misfolded proteins (amyloids) lead to the destruction of normal tissue structure and cellular dysfunction.
Rikinari Hanayama and colleagues from Kanazawa University have now found that extracellular vesicles (small biocontainers surrounded by a membrane) contribute to the aggregation and deposition of TTR amyloids in ATTRv amyloidosis.
[Article]
Title: Extracellular Vesicles Contribute to the Metabolism of Transthyretin Amyloid in Hereditary Transthyretin Amyloidosis
Journal: Frontiers in Molecular Biosciences
Authors: Hiroki Yamaguchi, Hironori Kawahara, Noriyuki Kodera, Ayanori Kumaki, Yasutake Tada, Zixin Tang, Kenji Sakai, Kenjiro Ono, Masahito Yamada, and Rikinari Hanayama.
DOI: 10.3389/fmolb.2022.839917



 PAGE TOP
PAGE TOP