Abstract: Researchers from Kanazawa University have shown that the chemokine CCL3 plays a significant role in the development of nonalcoholic steatohepatitis (NASH) from simple fatty liver. They used mice modified to lack CCL3 to show that resident macrophages in the liver secrete CCL3 and attract more immune cells, resulting in liver inflammation. The amount of CCL3 correlated with the severity of liver disease in human patients. Blocking CCL3 could therefore be a potential treatment for NASH.
Kanazawa, Japan – Nonalcoholic fatty liver disease (NAFLD) is significant worldwide and growing in prevalence, but little is known about how it develops. Now, a research team at Kanazawa University has shown that a molecule called CCL3 plays a key role in its development.
CCL3 is a molecule called a chemokine, a small signaling protein that acts as an attractant for certain white blood cells. Other chemokines were already thought to be involved in the transition from simple fatty liver to nonalcoholic steatohepatitis, or NASH — characterized by fat in the liver alongside inflammation and damage to liver cells — but no role had been shown for CCL3.
The research team showed that the presence of CCL3 increased the accumulation of macrophages, a type of white blood cell, in the liver. They fed mice a high-cholesterol high-fat diet to induce NASH. The levels of macrophages secreting CCL3 in the liver increased as NASH developed.
The team then studied mice that had been modified to lack the CCL3 gene. Mice lacking CCL3 that were fed the high-cholesterol high-fat diet did not develop the same degree of liver inflammation as mice that could produce CCL3. The lack of CCL3 in mice that had been genetically modified to be obese also reduced the amount of associated liver inflammation and insulin resistance.
When liver macrophages sense damage, they secrete various chemokines to attract other immune cells and shift to an active state known as proinflammatory M1 phenotype. Their action causes liver inflammation and scarring. The researchers showed that these M1 macrophages were expressing CCL3. Mice lacking CCL3 showed a 48% reduction in the number of M1-phenotype macrophages in the liver compared with CCL3-producing mice after being fed the high-cholesterol high-fat diet.
“We also showed that CCL3 itself promoted the transition of macrophages to the M1 phenotype and suppressed them shifting to the alternative M2 phenotype, which is associated with tissue repair and wound healing,” says lead author Liang Xu. “This is, as far as we are aware, the first report of CCL3 affecting macrophage phenotype polarization.”
Finally, they took blood samples from patients with confirmed cases of NAFLD and compared the serum levels of CCL3 with those from healthy volunteers. “The serum CCL3 levels increased with the histological severity of liver pathology, such as ballooning, steatosis, and lobular inflammation,” explains senior author Naoto Nagata, “and patients with full-blown NASH showed the highest CCL3 levels.”
This study indicates that blocking the action of the CCL3 chemokine could be a potential treatment option for NASH, although further work is needed. This could help patients worldwide who are suffering from this liver disease.
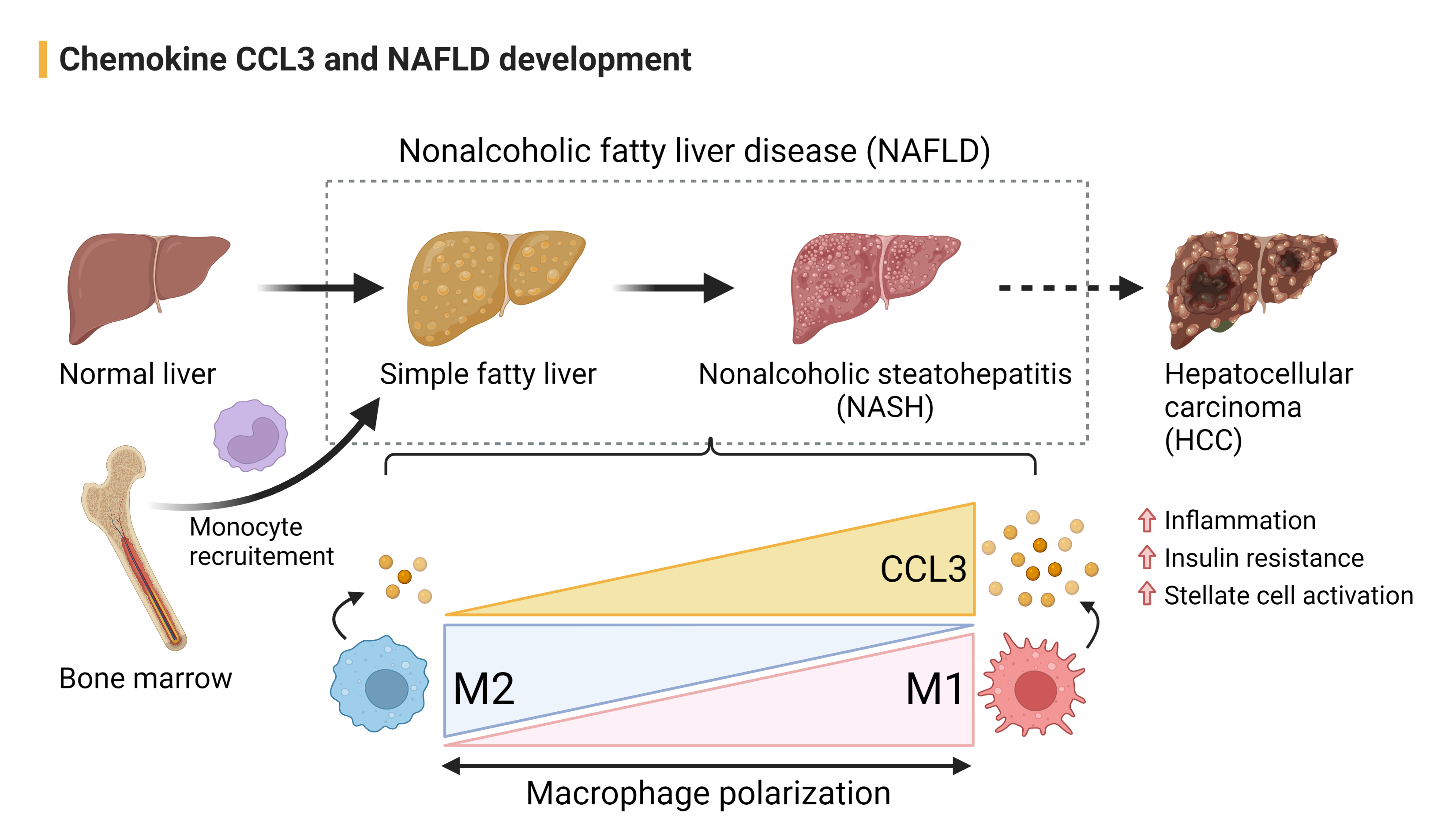
Figure 1.
Nonalcoholic fatty liver disease (NAFLD) is characterized by the accumulation of fat in ≥5% of hepatocytes in the absence of significant alcohol consumption (20 g/day for woman and 30 g/day for man). Some individuals with NAFLD can develop nonalcoholic steatohepatitis (NASH), a more severe case of fatty liver disease, which is marked by liver inflammation and fibrosis and may progress to cirrhosis and hepatocellular carcinoma (HCC). Chemokine CCL3 largely produced by pro-inflammatory M1 macrophages rather than alternatively activated M2 macrophages. In the development of NAFLD, CCL3 promotes 1) bone marrow-derived monocytes (BMDMs) recruitment into the inflamed liver and 2) M1-dominant liver macrophage polarization, which contribute to sustaining the inflammation resulting in insulin resistance and hepatic stellate cell activation.
[Article]
Title: C—C chemokine ligand 3 deficiency ameliorates diet-induced steatohepatitis by regulating liver macrophage recruitment and M1/M2 status in mice
Journal: Metabolism Clinical
Authors: Liang Xu, Yongping Chen, Mayumi Nagashimada, Yinhua Ni, Fen Zhuge, Guanliang Chen, Haoran Li, Tongtong Pan, Tatsuya Yamashita, Naofumi Mukaida, Shuichi Kaneko, Tsuguhito Ota, Naoto Nagata
DOI: 10.1016/j.metabol.2021.154914
[Founder]
This work was supported by a grant Grant-in-Aid for Scientific Research (B) (25282017), a grant Grant-in-Aid for Scientific Research (A) (26253046) and a grant for Challenging Exploratory Research (15K12698) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.



 PAGE TOP
PAGE TOP