- Home
- Highlights
- Understanding molecular rotation
Highlights
Understanding molecular rotation
Microscope videos recorded by Japanese researchers reveal how rotational forces are induced in a motor protein that is important for the cellular energy cycle.
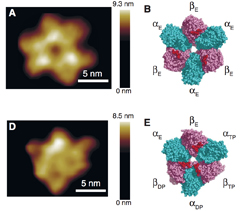
The research team, including Toshio Ando at Kanazawa University and Hiroyuki Noji of Osaka University and the University of Tokyo, used high-speed atomic force microscopy (HS-AFM) to capture videos of F1-ATPase, an abundant enzyme found in most living organisms. F1-ATPase comprises a hexagonal ring made from three each of so-called alpha and beta units, and a rotating central ‘gamma’ shaft structure. When the gamma shaft is rotated by an external force, interactions within the structure catalyze the production of adenosine tri-phosphate (ATP), often referred to as the unit of currency for energy transfer within and between cells. Conversely, ATP can be hydrolyzed within the beta units, acting as a fuel to drive the rotation.
Previous works have shown that the latter reaction, known as rotary catalysis, causes the three beta units to expand and contract in a sequence that makes the gamma shaft rotate. It was concluded that this motion relied on interactions between the gamma and beta parts. To test this theory, Ando, Noji and co-workers used HS-AFM to examine a ‘rotorless’ alpha-beta ring of F1-ATPase, which had the gamma shaft removed. By capturing images at 80 millisecond intervals, they found that the beta units still expanded and contracted in a counter-clockwise sequence - implying that interactions with the gamma shaft were not required to produce rotation.
These findings could have implications for understanding the interactions between subunits in other enzymes, and demonstrate the widespread benefits of the HS-AFM techniques pioneered by Ando and his team.
Publication and Affiliation
Takayuki Uchihashi1,2,3,* Ryota Iino3,4,5,* Toshio Ando1,2,3 & Hiroyuki Noji3,4,5,* High-speed atomic force
microscopy reveals rotary catalysis of rotorless F1-ATPase. Science, 333, 755-758, (2011)
Link
1. Department of Physics, Kanazawa University, Kakuma-machi, Kanazawa 920-1192, Japan
2. Bio–AFM Frontier Research Center, College of Science & Engineering, Kanazawa University, Kakuma-machi, Kanazawa 920-1192, Japan
3. Core Research for Evolutional Science and Technology, Japan Science and Technology Agency, Sanban-cho, Chiyoda-ku, Tokyo 102-0075, Japan
4. Institute of Scientific and Industrial Research, Osaka University, Ibaraki, Osaka 567-0047, Japan
5. Department of Applied Chemistry, School of Engineering, The University of Tokyo, Bunkyo-ku, Tokyo 113-8656, Japan
*corresponding authors, e-mail address: hnoji@appchem.t.u-tokyo.ac.jp, tando@staff.kanazawa-u.ac.jp
ID: 201212B002