Abstract:
Selenoprotein P, a kind of hepatokine hormone secreted from the liver, has been found, through experiments with cultured muscle cells and mice and through clinical studies, to cause pathology named ‘exercise resistance,’ which prevents health promotion by physical exercise. The present results elucidate one of the reasons why individual responsiveness to exercise differs markedly as well as shed lights on development of therapy for lifestyle diseases due to lack of exercise, obesity and type-2 diabetes.
[Background]
In Japan, due to the changes of lifestyle such as less physical activities than before, more and more people suffer from the lifestyle diseases like metabolic syndromes, type-2 diabetes and hypertension. Regular physical exercise is recommended as ‘exercise therapy’ since it will lead to prevention and therapy of the diseases mentioned above. However, individual responsiveness to exercise is known to differ markedly. Some people derive little benefit from the health promoting effects of regular exercise, which has been a big problem.
The researchers of Kanazawa University reported that selenoprotein P*1, a protein produced in and secreted from the liver, was high in terms of its concentration in the blood in type-2 diabetes patients and that selenoprotein P augmented the insulin resistance to induce elevation of blood glucose level (Cell Metabolism 2010; 12(5), 483). They proposed to call the hormone ‘hepatokine*2’ that was secreted from the liver, was delivered to various organs and tissues of the body by the blood and would exert diverse effects. However, the effects of selenoprotein P, a hepatokine, on health promotion by physical exercise was unclear.
[Results]
The present research team of Kanazawa University, two universities, a company and a Chinese hospital has demonstrated the followings through investigating the effects of selenoprotein P on the results of physical exercise by the experiments with mice and cultured muscle cells and by clinical studies. Mice were subjected to exercise training on a treadmill for 30 min per day during one month. The team has found that after the one month exercise, the selenoprotein P-deficient mice showed twice higher exercise capacity than the wild type (WT) mice.
After the training, the selenoprotein P-deficient mice also showed larger reduction in the blood glucose level upon insulin injection than the WT mice.
It was shown that with the WT mice administered with selenoprotein P, muscles after the one month training exhibited reduced level of AMPK*3 phosphorylation; AMPK phosphorylation is considered to be related with a variety of favorable training effects. Furthermore, it was shown that the mice deficient of LRP1*4, the receptor of selenoprotein P in muscles, did not incorporate administered selenoprotein P into muscles and that AMPK phosphorylation upon training was not affected.
A total of healthy but sedentary 31 women without obesity or type-2 diabetes underwent aerobic training for 8 weeks, and maximal oxygen consumption was measured as exercise capacity. In general, the maximal oxygen consumption was elevated after the training, whereas some women did not show much elevation. Those women had high level of selenoprotein P in the blood before the training.
These results demonstrate that selenoprotein P causes ‘exercise resistance*5’ by affecting muscles through the receptor LRP1, hence cancelling the effects of exercise.
[Significance]
It has been reported that the selenoprotein P level in the blood is high in patients with type-2 diabetes or fatty liver and in persons at high age. There is a possibility that because of excess level of selenoprotein P, those patients and persons suffer from exercise resistance and derive limited benefits from the heath-promoting effects of physical exercise.
The results of the present research are expected to lead to discovery of ‘drugs to promote exercise effects’ through the search for drugs reducing selenoprotein P production in the liver and for others competing with LRP1, the selenoprotein P receptor of muscles.
It is also expected that individual persons could be diagnosed to be exercise-effective or exercise-ineffective by measuring selenoprotein P level in the blood.
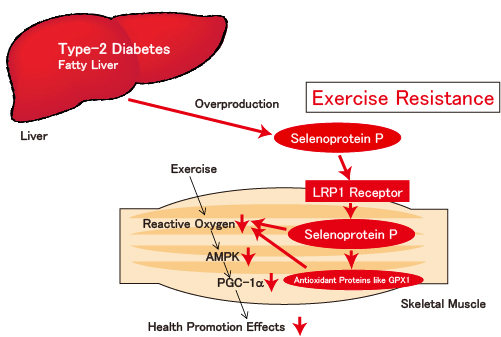
Figure: Selenoprotein P, a hepatokine secreted from the liver, exerts its effects upon binding to LRP1 receptor of muscles, inducing exercise resistance.
In patients with type-2 diabetes and in those with fatty liver, overproduced selenoprotein P exerts its effects on muscles through its binding to LRP1 receptor. Selenoprotein P incorporated into muscles induces antioxidant proteins such as GPX1 and SeW, As the result, the level of reactive oxygen produced by physical exercise is suppressed, and those patients suffer from pathology of ‘exercise resistance’ due to low responsiveness to physical exercise.
[Glossary]
*1 Selenoprotein P
Selenoprotein P is a secretory protein, produced mainly in the liver. Since it contains high level of selenium, a minor but essential element, it was considered to be a selenium-transferring hormone from the liver to other tissues and organs.
However, the researchers of Kanazawa University demonstrated in 2010 that the concentration of selenoprotein P in the blood was elevated in type-2 diabetes patients and that selenoprotein P functioned as a hormone to increase the blood glucose level. Recently, it has been reported that the blood level of selenoprotein P is elevated in patients with fatty liver and in persons at high age.
*2 Hepatokine
Generic name of a group of hormones secreted from the liver. Hepatokine exerts diverse effects at various tissues and organs of the body after being conveyed by the blood.
The researchers identified selenoprotein P in 2010, a humoral factor from the liver causing hyperglycemia. Selenoprotein P was found to be elevated in terms of its concentration in the blood in type-2 diabetes. The researchers named such hormone from the liver as hepatokine.
*3 AMPK
AMPK, abbreviation of AMP-activated protein kinase, is one of the enzymes in the cell. It is known that exercise causes phosphorylation of AMPK in muscle cells and AMPK activation. It has been reported that the activation of AMPK brings about benefits such as increasing the number of mitochondria in the cell, increasing incorporation of glucose into the cell, increasing insulin sensitivity and so on.
*4 LRP1
LRP1, abbreviation of low density lipoprotein receptor-related protein 1, is a protein in the cell membrane. It has been reported to play roles in uptake of ligands such as cholesterol and blood coagulation factors as well as in signaling pathways. However, the research team has found, for the first time, that LRP1 is involved in the effects of exercise and in the uptake of selenoprotein P.
*5 Exercise resistance
Physical exercise has health-promoting effects in general, but it has also been known that individual responsiveness to exercise differs significantly. The present research has indicated that individuals with high level of selenoprotein P in the blood show low responsiveness to exercise. The research team proposes to refer to this pathology as ‘exercise resistance.’
Article
Title: Deficiency of the hepatokine selenoprotein P increases responsiveness to exercise in mice through upregulation of reactive oxygen species and AMP-activated protein kinase in muscle
Journal: Nature Medicine
Authors: Hirofumi MISU1, Hiroaki TAKAYAMA1, Yoshiro SAITO2, Yuichiro MITA2, Akihiro KIKUCHI1, Kiyo-aki ISHII1, Keita CHIKAMOTO1, Takehiro KANAMORI1, Natsumi TAJIMA1, Fei LAN3, Yumie TAKESHITA1, Masao HONDA1, Mutsumi TANAKA4, Seiji KATO4, Naoto MATSUYAMA4, Yuya YOSHIOKA2, Kaito IWAYAMA5, Kumpei TOKUYAMA5, Nobuhiko AKAZAWA5, Seiji MAEDA5, Kazuhiro TAKEKOSHI5, Seiichi MATSUGO1, Noriko NOGUCHI2, Shuichi KANEKO1, Toshinari TAKAMURA1
1Kanazawa University, 2Doshisha University, 3Chengdu First People’s Hospital, China, 4Alfresa Pharma Corporation, 5University of Tsukuba
Doi: 10.1038/nm.4295
Funder
JSPS KAKENHI 25461334, 25292078, 16K09740, 26461329 and 26253046; Mochida Memorial Foundation for Medical and Pharmaceutical Research; Takeda Science Foundation; MEXT-Supported Program for the Strategic Research Foundation at Private Universities in Japan; JST A-STEP AS2311400F and 15im0302407



 PAGE TOP
PAGE TOP